Purpose of This Guideline
Date of current publication: May 6, 2024
Lead author: Jacob R. McLean, DO; Jason E. Zucker, MD
Writing group: Rona M. Vail, MD, AAHIVS; Sanjiv S. Shah, MD, MPH, AAHIVM, AAHIVS; Steven M. Fine, MD, PhD; Joseph P. McGowan, MD, FACP, FIDSA, AAHIVS; Samuel T. Merrick, MD, FIDSA; Asa E. Radix, MD, MPH, PhD, FACP, AAHIVS; Jessica Rodrigues, MPH, MS; Christopher J. Hoffmann, MD, MPH, MSc, FACP; Brianna L. Norton, DO, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: May 6, 2024
This guideline was developed by the New York State Department of Health AIDS Institute (NYSDOH AI) to inform primary care providers and other clinicians in New York State about mpox prevention, presentation, diagnosis, and treatment in adults with and without HIV. The goals of this guideline include:
- Increase clinicians’ awareness of and ability to recognize the common clinical manifestations of mpox and diagnose the disease in their patients.
- Provide clinicians with evidence-based recommendations on primary prevention, diagnostic testing, and supportive care and treatment of mpox.
- Increase clinicians’ awareness of recommended precautions to reduce occupational exposure to and community transmission of mpox.
Terminology: In November 2022, the Centers for Disease Control and Prevention (CDC) aligned its terminology with that of the World Health Organization and adopted the term mpox to refer to monkeypox, to reduce negative effects, including stigma, associated with the use of the term monkeypox. Mpox refers to the disease caused by infection with the human monkeypox virus (MPXV), a member of the Orthopoxvirus genus related to the smallpox virus, but the use of the 2 terms and associated abbreviations is not standardized. In this document, the term mpox is used to refer to the virus and the associated disease.
Epidemiology
Mpox was first described in 1970 in the Democratic Republic of Congo and subsequently caused sporadic outbreaks primarily in endemic areas of Central and West Africa. Beginning in May 2022, an outbreak of mpox initially identified in the United Kingdom spread globally, becoming the largest outbreak of this disease to date Laurenson-Schafer, et al. 2023.
The mpox virus species is subdivided into clades I and II, with clade II further subdivided into IIa and IIb. Clade IIb has driven the most recent mpox outbreak CDC(a) 2024 and appears to cause less severe symptoms than clade I in animal models Americo, et al. 2023.
Globally and in the United States, the recent mpox outbreak has affected primarily men who have sex with men (MSM) McQuiston, et al. 2023, although infections have occurred in people of all sexual orientations, genders, and ages. People with HIV have been disproportionately affected, comprising approximately 38% of U.S. mpox cases Curran, et al. 2022.
On August 4, 2022, the U.S. Department of Health and Human Services declared the mpox outbreak a public health emergency but allowed the declaration to expire in January 2023 when cases declined DHHS 2022. Available evidence suggests that the decline in cases was driven by the combined effect of behavior modification and vaccine uptake among people vulnerable to mpox Clay, et al. 2024; Moschese, et al. 2024; Paredes, et al. 2024; Zhang, et al. 2024. At the time of this publication, mpox incidence has remained low, but cases continue to be reported daily, suggesting endemicity. The CDC has warned about the potential for recurrent mpox outbreaks, especially among MSM in areas or networks with a low prevalence of immunity from prior infection or vaccination Pollock, et al. 2023. The CDC also has advised of a significant uptick in clade I mpox in the Democratic Republic of Congo from 2023 to 2024, including in sexual networks. Surveillance for clade I mpox virus is ongoing in the United States, and instructions about how to report possible clade I mpox are noted below CDC(a) 2023.
| KEY POINTS |
|
Transmission
Human-to-human transmission of mpox clade IIb occurs primarily via physical contact with an infected individual’s skin, saliva, or mucous membranes. In the 2022 mpox outbreak, sexual and other intimate contact is thought to have been the primary, although not exclusive, driver of viral spread McQuiston, et al. 2023; Tarin-Vicente, et al. 2022; Vaughan, et al. 2022. Transmission via fomites, usually soft, porous items such as linens, is less common, and respiratory transmission is theoretically possible but has not been formally reported CDC(e) 2024; Beeson, et al. 2023.
The incubation period for mpox ranges from 3 to 17 days, with a mean of approximately 6 days Madewell, et al. 2023, and illness may last from 2 to 4 weeks CDC(b) 2023; Madewell, et al. 2023. Fortunately, mortality from mpox is low, with only 56 deaths noted among the 31,689 mpox cases reported to the CDC as of January 10, 2024 CDC(f) 2024. Notably, of those who died for whom additional case data were available, 94% were immunocompromised due to advanced HIV Riser, et al. 2023.
Replication-competent virus has been detected for up to 3 weeks from symptom onset in immunocompetent individuals, with the highest burden and longest persistence of replication-competent virus found in skin lesions Palich, et al. 2023; Suner, et al. 2023. No cases of mpox transmission have occurred after skin lesions healed. Although there have been cases of presymptomatic transmission CDC(c) 2023; Miura, et al. 2023, the role of transmission in asymptomatic individuals remains unclear Mizushima, et al. 2023. The risk of transmission of mpox to healthcare professionals appears low, with the majority of events related to needlestick injuries sustained during attempts to unroof vesicles or lesions Choi, et al. 2023; Caldas, et al. 2022; Carvalho, et al. 2022; Mendoza, et al. 2022; Zachary and Shenoy 2022.
See Box 1, below, for an overview of the clinical presentation of mpox and strategies for preventing transmission and controlling infection, and see the guideline section Mpox Presentation and Diagnosis for an in-depth discussion of these topics.
| Abbreviation: CDC, Centers for Disease Control and Prevention. | |
| Box 1: Overview of Mpox Clinical Presentation, Transmission Prevention, and Infection Control | |
| Mpox Clinical Presentation | Transmission Prevention and Infection Control |
|
Healthcare providers: Use of personal protective equipment, including a gown, gloves, eyewear, and an N-95 or comparable respirator mask, will help prevent occupational exposure in healthcare providers who are evaluating or collecting a specimen from a patient with suspected mpox. There is no need to unroof lesions before swabbing, and this practice may increase the risk of needlestick injury and occupational infection CDC(c) 2024.
Patients: Although, ideally, patients with mpox will isolate at home and remain separate from other people, this may not always be feasible. To reduce the risk of community transmission, advise patients with confirmed or suspected mpox to do the following until all lesions have healed and other symptoms have resolved:
See CDC Mpox > Isolation and Infection Control at Home for more information, including disinfection strategies. |
Mpox Prevention
| RECOMMENDATIONS |
Mpox Prevention
|
Abbreviations: EUA, emergency use authorization; FDA, U.S. Food and Drug Administration; MVA, modified vaccinia Ankara (brand name JYNNEOS); PEP, post-exposure prophylaxis. Note:
|
As noted above, behavior changes and vaccine uptake are thought to have driven the rapid decline in mpox cases that occurred in the summer of 2022, underscoring the importance of patient education to promote prevention among those vulnerable to mpox. The sections below contain information related to mpox risk and MVA vaccine efficacy, safety, and use as PEP, all of which can help healthcare providers counsel their patients about mpox prevention.
Immunization
Primary prevention through immunization is a cornerstone of mpox epidemic control. The currently recommended mpox vaccine, brand name JYNNEOS, is based on MVA, a nonreplicating live virus vaccine originally developed as part of the global smallpox eradication effort. MVA is incapable of replication within human hosts, and because it is nonreplicating, exposure to MVA cannot result in infection, unlike prior versions of the smallpox vaccine.
The MVA vaccine is approved by the FDA for use in people ≥18 years old. In August 2022, the FDA issued an EUA for emergency use of the JYNNEOS vaccine in individuals <18 years old. Although clinical efficacy data in this population are not yet available, the vaccine has been shown to be safe and immunogenic Ladhani, et al. 2023. When considering vaccination of infants younger than 6 months old, clinicians should contact their jurisdictional health department.
Mpox immunization for primary prevention is recommended for individuals at elevated risk of infection, including but not limited to those in the groups listed in Box 2, below. Following the commercialization of the MVA vaccine, all individuals requesting vaccination or who believe they may be vulnerable to mpox can be considered for vaccination. Estimates of vaccine efficacy range from 36% to 86% for 1 dose and increase to 66% to 89% two weeks after administration of the second dose or completion of the 2-dose series Bertran, et al. 2023; Dalton, et al. 2023; Deputy(a), et al. 2023; Deputy(b), et al. 2023; Rosenberg, et al. 2023; Wolff Sagy, et al. 2023. Reports of breakthrough mpox in fully vaccinated individuals suggest that symptoms may be milder than in those with no preexisting immunity Hazra, et al. 2024; Farrar, et al. 2022. Infection with the human mpox virus generates a robust immune response Agrati, et al. 2023. At the time of publication, mpox immunization is not advised for individuals who have had prior laboratory-confirmed mpox. People reporting a history of symptoms consistent with mpox but without confirmatory testing should still be offered vaccination if otherwise indicated.
Administration: MVA is licensed for subcutaneous administration in a 2-dose series, with injections spaced 28 days apart. If the second dose is not given at 28 days, it should be administered as soon as possible thereafter. If the second dose is given less than 4 days early, the vaccine series does not need to be repeated CDC(d) 2024. Intradermal vaccination was used in the context of product shortages during the 2022 epidemic and appeared to generate antibody responses comparable to subcutaneous injection Brooks, et al. 2022 but was also associated with a higher risk of local cutaneous adverse reactions and potentially lasting hyperpigmentation Frey, et al. 2023; Frey, et al. 2015. For this reason, subcutaneous administration is the preferred route when supplies allow. Intradermal administration is not approved under the EUA for individuals younger than 18 years old.
Individuals with HIV: Given the disproportionate burden of mpox among people with HIV Curran, et al. 2022, vaccination of those at risk is a priority. Of note, although other smallpox vaccine products may confer some protection against mpox, MVA is the only vaccine that is safe for use in people with HIV CDC(b) 2024 and appears to have reliable immunogenicity in individuals without advanced immunocompromise Overton, et al. 2020; Greenberg, et al. 2013. Although MVA is technically a live vaccine, the virus does not replicate in human hosts and is not contraindicated in individuals with advanced HIV. Little is known about the immunogenicity of the MVA vaccine in people with HIV and advanced immunosuppression, but mpox vaccination should be offered regardless of immune status.
Individuals who are pregnant or breastfeeding: Limited data are available regarding the safety or associated risks of the MVA vaccine for individuals who are pregnant or breastfeeding. However, animal studies have found no risk to a developing fetus, and the replication-deficient nature of the MVA virus means there should be no risk of infection in breastfed infants Rao, et al. 2022. Healthcare providers may offer mpox vaccination as primary prophylaxis or PEP after engaging pregnant or breastfeeding individuals in shared decision-making that includes evaluation of known risks versus benefits.
| Box 2: Centers for Disease Control and Prevention Recommendations for Mpox Vaccination |
Mpox vaccination should be offered to:
|
Post-Exposure Prophylaxis
When administered as PEP within 14 days of mpox exposure, the MVA vaccine reduced the chance of symptomatic infection and symptom severity Montero Morales, et al. 2023. Precise estimates of the efficacy of this strategy are lacking, given the bias inherent in retrospective analysis Rosen, et al. 2024; Deputy(b), et al. 2023. Future research may answer this question Luong Nguyen, et al. 2022. Despite these limitations, asymptomatic individuals without prior immunity who have been exposed to mpox in the last 14 days should be offered vaccination as PEP, ideally within 4 days after exposure, to reduce the risk of infection or decrease symptoms. Individuals receiving vaccine PEP should be encouraged to complete the full vaccine series even in the absence of symptoms.
Mpox Presentation and Diagnosis
| RECOMMENDATIONS |
Mpox Presentation and Diagnosis
|
Abbreviations: NAAT, nucleic acid amplification testing; PCR, polymerase chain reaction; STI, sexually transmitted infection. Note:
|
Symptoms
Systemic symptoms including fever, headache, myalgias, lymphadenopathy, and malaise were commonly described as the presenting symptoms in mpox cases before the 2022 outbreak Titanji, et al. 2022; Ogoina, et al. 2020. Although most people with mpox will experience systemic symptoms at some point in the course of their infection, rash has been the initial manifestation in approximately 50% of recent cases Mailhe, et al. 2023; Philpott, et al. 2022. Lymphadenopathy localized around sites of mucosal involvement is more common than generalized lymphadenopathy.
Rash: Mpox is characterized by lesions on the skin and mucous membranes. During the 2022 mpox outbreak, rash was observed most often in the anogenital area but was also found on the mouth, hands, face, feet, or chest Philpott, et al. 2022; Thornhill, et al. 2022. Lesions classically progress from macule to papule to pustule or vesicle before crusting and, ultimately, healing with new skin formation in 2 to 4 weeks. During the 2022 mpox outbreak, it was common for patients to have lesions in multiple stages on the same body part at the same time CDC(b) 2023.
The most typical lesions are pustules or vesicles that are often umbilicated, deep-seated, and painful. Rash extent varies considerably, ranging from a single lesion to disseminated disease. Open lesions can develop bacterial superinfection, resulting in cellulitis or abscess in the surrounding skin. Figure 1, below, shows examples of characteristic lesions.
Figure 1: Stages of Mpox Lesions
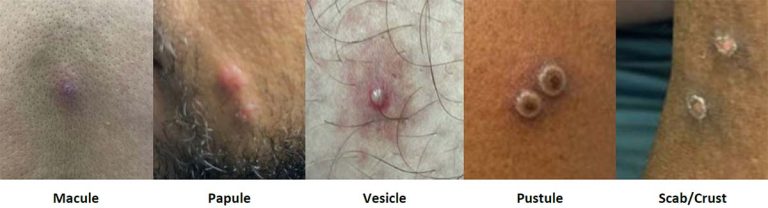
Notes:
- Photographs collected by the authors with patient consent.
- See also Centers for Disease Control and Prevention Mpox > Clinical Recognition.
Download figure: Stages of Mpox Lesions
Mucosal involvement: Mucosal involvement may occur at the site of exposure and is responsible for much of the morbidity of mpox Tarin-Vicente, et al. 2022. Proctitis was noted in 20% to 30% of cases in the 2022 mpox outbreak Cassir, et al. 2022; Català, et al. 2022; Tarin-Vicente, et al. 2022 and may be present without visible perianal lesions. Typical symptoms of pain, tenesmus, and discharge may be accompanied by diarrhea or constipation. Oropharyngeal involvement, which has been noted in 22% of cases, is also common and may lead to odynophagia that interferes with eating or drinking Gandhi, et al. 2023; Shah, et al. 2023.
Ocular disease: Ophthalmologic manifestations, including keratitis, conjunctivitis, or blepharitis, may occur through autoinoculation and can cause lasting vision impairment Abdelaal, et al. 2023; Cash-Goldwasser, et al. 2022.
Severe disease: Severe necrotizing mpox is more common in immunocompromised individuals, including those with advanced HIV. Severe disease may include a higher lesion burden and other organ involvement, such as pneumonitis or encephalitis. Of note, individuals with well-controlled HIV present with symptoms similar to those in individuals without HIV McLean, et al. 2023.
| KEY POINT |
|
STI Coinfection and Mpox Differential Diagnoses
Many patients presenting with mpox symptoms will have other STIs that may have overlapping symptoms. Herpes simplex virus lesions can be particularly difficult to distinguish from mpox lesions. Obtaining a detailed sexual history, considering additional or alternative processes, and offering STI screening when indicated are critical when evaluating a patient with suspected mpox.
Table 1, below, outlines common differential diagnoses based on clinical syndrome and features that may distinguish mpox from other infections. The British Medical Journal (BMJ) also provides a comprehensive list of infectious and noninfectious differentials: BMJ Best Practice > Mpox Diagnosis > Differentials.
| Table 1: Common Differential Diagnoses for Clinical Syndromes Caused by Mpox | |
| Clinical Syndrome | Common Differential Diagnoses and Distinguishing Features |
| Rash, localized or general |
|
| Genital ulcer |
|
| Proctitis |
|
Mpox Diagnostic Testing
Mpox is diagnosed via PCR analysis of skin lesion specimens. Whenever possible, to maximize sensitivity, 2 specimens should be collected from each of 2 separate lesions, preferably in different stages and at different body sites (4 swabs in total). When feasible, sanitize the patient’s skin with an alcohol wipe and allow the skin to air dry. Rub swabs vigorously on the base of the lesion to ensure adequate transfer of cells onto the swab surface. Lesions should not be unroofed before swabbing because this practice may increase the risk of needlestick injury and occupational infection CDC(c) 2024.
If a patient has no skin lesions, mpox virus may be detected in other compartments such as the throat or rectum, although testing at these sites is not approved by the U.S. Food and Drug Administration. In patients with skin lesions, testing additional sites does not increase the chance of diagnosis because skin lesions have the highest viral loads and longest clearing time Palich, et al. 2023; Suner, et al. 2023.
Synthetic swabs (not cotton) can be submitted dry or in viral or universal transport media. Bacterial transport media should be avoided because this can interfere with PCR assays. Depending on the laboratory facility used, crusts taken from lesions may also be acceptable specimens. Confirm requirements with the laboratory facility processing the specimen. Many commercial laboratories offer mpox testing, as do the NYSDOH Wadsworth Center and New York City Public Health Laboratory.
Anyone with suspected or diagnosed mpox potentially contracted via sexual contact should receive HIV antibody/antigen testing, syphilis serologies, and gonorrhea and chlamydia NAAT of the urine, cervix, rectum, or pharynx depending on site(s) of exposure. See the NYSDOH AI guideline HIV Testing.
In response to an ongoing outbreak of clade I mpox virus in the Democratic Republic of Congo (DRC), the Centers for Disease Control and Prevention (CDC) recommend that individuals with suspected mpox who have traveled to the DRC within the previous 21 days undergo clade-specific testing CDC(a) 2023. For consultation about testing and treatment of such individuals, care providers in New York City can call the Provider Access Line at 1-866-692-3641; care providers in other counties in New York State can call the Office of Sexual Health and Epidemiology at 1-518-474-3598 during business hours or 1-866-881-2809 during evenings, weekends, and holidays. See the NYSDOH December 12, 2023 Dear Colleague Letter for additional information.
See NYSDOH Guidance on Testing at Commercial and Public Health Laboratories and CDC Guidelines for Collecting and Handling Specimens for Mpox Testing for additional guidance on best practices for mpox specimen collection.
| NEW YORK STATE LAW |
Per New York State Public Health Law, all positive mpox test results must be reported to the local health department. See the following for more information: |
Transmission Prevention and Infection Control
Preventing occupational mpox exposure: Healthcare providers should practice effective hand hygiene and don personal protective equipment (gown, gloves, eyewear, and an N-95 or comparable respirator mask) before evaluating or collecting a specimen from a patient with suspected mpox. See the guideline section Purpose of This Guideline > Transmission for discussion of routes of potential mpox exposure.
Preventing community mpox transmission: Healthcare providers should recommend that patients with mpox take the following precautions to protect others from exposure and prevent transmission:
- Isolate in the home if feasible
- Avoid skin-to-skin and sexual contact
- Avoid sharing clothing, bed linens, and other soft, porous materials that may have come into contact with a lesion
- Avoid sharing eating or personal hygiene utensils, such as razors; if items must be shared, wash and disinfect after each use
- Avoid exposing other people to lesions when in public or shared spaces; cover all lesions with clothing, bandages, or gloves
- Wear a medical mask if in close proximity with other people for more than a brief encounter (per the CDC)
In individuals with suspected mpox, the above precautions should be continued until mpox has been ruled out. For those with confirmed mpox, precautions should be continued until all lesions have crusted, crusts have separated, and a new layer of skin has formed underneath.
See CDC Mpox > Isolation and Infection Control at Home for more information, including disinfection strategies.
Mpox Treatment
Fortunately, the prognosis of mpox in the context of the 2022 clade IIb mpox outbreak is excellent, and the majority of affected individuals recover fully whether they receive medical attention or not CDC(f) 2024. Supportive measures for pain and other symptom control as well as treatment of complications, such as bacterial superinfection, are the mainstays of therapy. Although there is no U.S. Food and Drug Administration (FDA)-approved therapy specifically for mpox, several antiviral medications developed for the treatment of infection with other pathogens have been repurposed as mpox medical countermeasures.
Supportive Care
Many patients with mpox will experience significant pain from skin lesions or mucosal involvement, including proctitis or pharyngitis. Although there is limited empirical evidence, the Centers for Disease Control and Prevention (CDC) has provided clinical considerations for supportive care and pain management of mpox based on the clinical experience of healthcare providers. Pain can often be controlled with over-the-counter analgesics such as acetaminophen or nonsteroidal anti-inflammatory medications. Some individuals may require treatment with gabapentin or opioid medications for severe pain. For patients with opioid use disorder on medication-assisted treatment, consider recommendations available in the U.S Department of Veteran’s Affairs Evidence Brief: Managing Acute Pain in Patients with Opioid Use Disorder on Medication-assisted Treatment.
Topical therapies such as sitz baths for proctitis and saltwater or viscous lidocaine gargles for pharyngitis can also be used. Stool softeners can offer relief for painful defecation with proctitis and can also be considered for patients treated with opioids. When bacterial superinfection of mpox skin lesions is suspected, the recommended treatment is topical or systemic antibiotics as per usual for skin and soft tissue infections. Table 2, below, outlines supportive care measures for complications associated with mpox.
| Table 2: Supportive Care Measures for Mpox Complications | ||
| Proctitis | Pharyngitis | Genital lesions |
|
|
|
Medical Countermeasures
Tecovirimat: Tecovirimat has been the preferred antiviral agent during the 2022 mpox outbreak. It was originally developed for the treatment of smallpox (caused by variola, another orthopoxvirus) and approved by the FDA in 2018 through the animal rule based on animal efficacy studies. This medication acts by targeting the p37 protein, a component of the viral envelope in the mpox virus and related orthopoxviruses DeLaurentis, et al. 2022. Tecovirimat was made available through an expanded access investigational new drug (EA-IND) protocol and has been provided to more than 6,800 people during the 2022 mpox outbreak. To date, only mild adverse effects have been reported in safety data [O’Laughlin, et al. 2022], with mixed results on efficacy in observational data Akiyama, et al. 2024; Karmarkar, et al. 2024; Mazzotta, et al. 2023. Surveillance data demonstrated the development of resistance in immunocompromised individuals requiring extended courses of tecovirimat, and presumed transmitted resistance has been identified in individuals not exposed to tecovirimat Garrigues(a), et al. 2023; Garrigues(b), et al. 2023.
Clinicians are encouraged to inform all individuals with presumed or confirmed mpox about the NIH STOMP study, which is the primary means of access to tecovirimat. This study, underway since September 2022, is a placebo-controlled double-blind clinical trial with a 2:1 randomization scheme designed to measure the effect of tecovirimat on time to mpox resolution. Individuals who have or are at risk of developing severe mpox, children, and individuals who are pregnant or breastfeeding are allocated to an open-label study arm and will receive tecovirimat. Remote enrollment is available for those not near a study site. The call center listed at the link above can direct healthcare providers to nearby study sites or options for remote enrollment.
Patients who do not wish to or are unable to participate in the NIH STOMP study and patients who require intravenous tecovirimat may be eligible to receive the medication through the CDC EA-IND protocol. This is available only to individuals who have or are at risk of developing severe disease. Care providers should work in concert with their county health department to request a supply of tecovirimat by calling the CDC Emergency Operations Center at 770-488-7100 or emailing poxvirus@cdc.gov.
Cidofovir and brincidofovir: Cidofovir and its derivative, brincidofovir, are antiviral drugs that block DNA polymerase, thus stopping further DNA synthesis and leading to nonproductive infection. Cidofovir is approved by the FDA for intravenous treatment of cytomegalovirus retinitis. Animal studies suggest cidofovir might be effective against orthopoxviruses, but there are no human data yet to confirm its effectiveness in treating mpox. Because of the risk of cidofovir-associated kidney damage, its intravenous form is typically reserved for severe cases of mpox Rao, et al. 2023, especially in patients with significant immunosuppression. Topical cidofovir has been used as a cream or injected directly into lesions, and case reports have noted improvements when cidofovir was used in this way Buechler, et al. 2023. Brincidofovir is thought to be less harmful to the kidneys but may lead to adverse effects such as diarrhea and liver damage. Animal studies suggest a synergistic effect between brincidofovir and tecovirimat Quenelle, et al. 2007. Brincidofovir is also approved via the animal rule and is available as an EA-IND for mpox treatment.
Vaccinia immune globulin intravenous (VIGIV): VIGIV treatment involves the administration of antibodies targeting the vaccinia virus and is thought to offer some protection against mpox Rao, et al. 2023. This therapy can be particularly advantageous for individuals with compromised immune systems, such as those with advanced HIV, who may be unable to produce an adequate antibody response to infection Thet, et al. 2023.
Use of cidofovir, brincidofovir, and vaccine immunoglobulins has been limited to patients with severe disease and should occur only in consultation with an experienced specialist or the CDC Clinical Consultation Team, available by email at poxvirus@CDC.gov.
Information on how to access all the medical countermeasures discussed above can be found at CDC Treatment Information for Healthcare Professionals.
Trifluridine: The topical antiviral agent trifluridine has in vitro activity against orthopoxviruses and is approved by the FDA for treatment of eye infections caused by herpes simplex virus Cinatl, et al. 2024; Pepose, et al. 2003; Hyndiuk, et al. 1976. Although efficacy for ocular mpox has not been established, trifluridine was used in the 2022 outbreak with anecdotal success Perzia, et al. 2023. Trifluridine can be offered to patients with mpox ocular disease, preferably in consultation with an ophthalmologist.
All Recommendations
| ALL RECOMMENDATIONS: PREVENTION AND TREATMENT OF MPOX |
Mpox Prevention
Mpox Presentation and Diagnosis
|
Abbreviations: EUA, emergency use authorization; FDA, U.S. Food and Drug Administration; MVA, modified vaccinia Ankara (brand name JYNNEOS); NAAT, nucleic acid amplification testing; PEP, post-exposure prophylaxis; PCR, polymerase chain reaction; STI, sexually transmitted infection. Notes:
|
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making

Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Abdelaal A., Serhan H. A., Mahmoud M. A., et al. Ophthalmic manifestations of monkeypox virus. Eye (Lond) 2023;37(3):383-85. [PMID: 35896700]
Agrati C., Cossarizza A., Mazzotta V., et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect Dis 2023;23(3):320-30. [PMID: 36356606]
Akiyama Y., Morioka S., Tsuzuki S., et al. Efficacy and viral dynamics of tecovirimat in patients with MPOX: a multicenter open-label, double-arm trial in Japan. J Infect Chemother 2024;30(6):488-93. [PMID: 38042298]
Americo J. L., Earl P. L., Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A 2023;120(8):e2220415120. [PMID: 36787354]
Beeson A., Styczynski A., Hutson C. L., et al. Mpox respiratory transmission: the state of the evidence. Lancet Microbe 2023;4(4):e277-83. [PMID: 36898398]
Bertran M., Andrews N., Davison C., et al. Effectiveness of one dose of MVA-BN smallpox vaccine against mpox in England using the case-coverage method: an observational study. Lancet Infect Dis 2023;23(7):828-35. [PMID: 36924787]
Brooks J. T., Marks P., Goldstein R. H., et al. Intradermal vaccination for monkeypox - benefits for individual and public health. N Engl J Med 2022;387(13):1151-53. [PMID: 36044621]
Buechler C. R., Anderson Z., Kullberg S. A., et al. Successful treatment of recalcitrant mpox lesions with intralesional cidofovir in a patient with HIV/AIDS. JAMA Dermatol 2023;160(2):235-36. [PMID: 38055229]
Caldas J. P., Valdoleiros S. R., Rebelo S., et al. Monkeypox after occupational needlestick injury from pustule. Emerg Infect Dis 2022;28(12):2516-19. [PMID: 36252152]
Carvalho L. B., Casadio L. V. B., Polly M., et al. Monkeypox virus transmission to healthcare worker through needlestick injury, Brazil. Emerg Infect Dis 2022;28(11):2334-36. [PMID: 36121391]
Cash-Goldwasser S., Labuda S. M., McCormick D. W., et al. Ocular monkeypox - United States, July-September 2022. MMWR Morb Mortal Wkly Rep 2022;71(42):1343-47. [PMID: 36264836]
Cassir N., Cardona F., Tissot-Dupont H., et al. Observational cohort study of evolving epidemiologic, clinical, and virologic features of monkeypox in Southern France. Emerg Infect Dis 2022;28(12):2409-15. [PMID: 36241422]
Català A., Clavo-Escribano P., Riera-Monroig J., et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol 2022;187(5):765-72. [PMID: 35917191]
CDC(a). Mpox caused by human-to-human transmission of monkeypox virus with geographic spread in the Democratic Republic of the Congo. 2023 Dec 7. https://emergency.cdc.gov/han/2023/han00501.asp [accessed 2024 Mar 28]
CDC(a). About mpox. 2024 Apr 18. https://www.cdc.gov/poxvirus/mpox/about/index.html [accessed 2024 Feb 25]
CDC(b). Mpox: clinical recognition. 2023 Aug 30. https://www.cdc.gov/poxvirus/mpox/clinicians/clinical-recognition.html
CDC(b). Clinical considerations for treatment and prophylaxis of mpox infection in people who are immunocompromised. 2024 Apr 15. https://www.cdc.gov/poxvirus/mpox/clinicians/people-with-HIV.html [accessed 2024 Feb 15]
CDC(c). Science brief: detection and transmission of mpox (formerly monkeypox) virus during the 2022 clade IIb outbreak. 2023 Feb 2. https://stacks.cdc.gov/view/cdc/124367 [accessed 2024 Feb 15]
CDC(c). Guidelines for collecting and handling specimens for mpox testing. 2024 Apr 29. https://www.cdc.gov/poxvirus/mpox/clinicians/prep-collection-specimens.html [accessed 2024 Feb 15]
CDC(d). Interim clinical considerations for use of JYNNEOS vaccine for mpox prevention in the United States. 2024 Apr 22. https://www.cdc.gov/poxvirus/mpox/clinicians/vaccines/vaccine-considerations.html [accessed 2024 Mar 28]
CDC(e). Mpox: how it spreads. 2024 Mar 1. https://www.cdc.gov/poxvirus/mpox/if-sick/transmission.html [accessed 2024 Feb 15]
CDC(f). Mpox: ongoing 2022 global outbreak cases and data. 2024 Mar 5. https://www.cdc.gov/poxvirus/mpox/response/2022/index.html [accessed 2024 Mar 1]
Choi Y., Jeon E. B., Kim T., et al. Case report and literature review of occupational transmission of monkeypox virus to healthcare workers, South Korea. Emerg Infect Dis 2023;29(5):997-1001. [PMID: 36856759]
Cinatl J., Bechtel M., Reus P., et al. Trifluridine for treatment of mpox infection in drug combinations in ophthalmic cell models. J Med Virol 2024;96(1):e29354. [PMID: 38180134]
Clay P. A., Asher J. M., Carnes N., et al. Modelling the impact of vaccination and sexual behaviour adaptations on mpox cases in the USA during the 2022 outbreak. Sex Transm Infect 2024;100(2):70-76. [PMID: 38050171]
Curran K. G., Eberly K., Russell O. O., et al. HIV and sexually transmitted infections among persons with monkeypox - eight U.S. jurisdictions, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71(36):1141-47. [PMID: 36074735]
Dalton A. F., Diallo A. O., Chard A. N., et al. Estimated effectiveness of JYNNEOS vaccine in preventing mpox: a multijurisdictional case-control study - United States, August 19, 2022-March 31, 2023. MMWR Morb Mortal Wkly Rep 2023;72(20):553-58. [PMID: 37200229]
DeLaurentis C. E., Kiser J., Zucker J. New perspectives on antimicrobial agents: tecovirimat for treatment of human monkeypox virus. Antimicrob Agents Chemother 2022;66(12):e0122622. [PMID: 36374026]
Deputy(a) N. P., Gerhart J. L., Feldstein L. R. Vaccine effectiveness against mpox in the United States. N Engl J Med 2023;389(15):1440-41. [PMID: 37819967]
Deputy(b) N. P., Deckert J., Chard A. N., et al. Vaccine effectiveness of JYNNEOS against mpox disease in the United States. N Engl J Med 2023;388(26):2434-43. [PMID: 37199451]
DHHS. Statement from HHS Secretary Becerra on mpox. 2022 Dec 2. https://www.hhs.gov/about/news/2022/12/02/statement-from-hhs-secretary-becerra-on-mpox.html [accessed 2024 Feb 15]
Farrar J. L., Lewis N. M., Houck K., et al. Demographic and clinical characteristics of mpox in persons who had previously received 1 dose of JYNNEOS vaccine and in unvaccinated persons - 29 U.S. jurisdictions, May 22-September 3, 2022. MMWR Morb Mortal Wkly Rep 2022;71(5152):1610-15. [PMID: 36580416]
Frey S. E., Goll J. B., Beigel J. H. Erythema and induration after mpox (JYNNEOS) vaccination revisited. N Engl J Med 2023;388(15):1432-35. [PMID: 36947462]
Frey S. E., Wald A., Edupuganti S., et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naive subjects. Vaccine 2015;33(39):5225-34. [PMID: 26143613]
Gandhi P. A., Patro S. K., Sandeep M., et al. Oral manifestation of the monkeypox virus: a systematic review and meta-analysis. EClinicalMedicine 2023;56:101817. [PMID: 36628187]
Garrigues(a) J. M., Hemarajata P., Espinosa A., et al. Community spread of a human monkeypox virus variant with a tecovirimat resistance-associated mutation. Antimicrob Agents Chemother 2023;67(11):e0097223. [PMID: 37823631]
Garrigues(b) J. M., Hemarajata P., Karan A., et al. Identification of tecovirimat resistance-associated mutations in human monkeypox virus - Los Angeles County. Antimicrob Agents Chemother 2023;67(7):e0056823. [PMID: 37338408]
Greenberg R. N., Overton E. T., Haas D. W., et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis 2013;207(5):749-58. [PMID: 23225902]
Hazra A., Zucker J., Bell E., et al. Mpox in people with past infection or a complete vaccination course: a global case series. Lancet Infect Dis 2024;24(1):57-64. [PMID: 37678309]
Hyndiuk R. A., Seideman S., Leibsohn J. M. Treatment of vaccinial keratitis with trifluorothymidine. Arch Ophthalmol 1976;94(10):1785-86. [PMID: 823931]
Karmarkar E. N., Golden M. R., Kerani R. P., et al. Association of tecovirimat therapy with mpox symptom improvement: a cross-sectional study-King County, Washington, May-October 2022. Open Forum Infect Dis 2024;11(3):ofae029. [PMID: 38456195]
Ladhani S. N., Dowell A. C., Jones S., et al. Early evaluation of the safety, reactogenicity, and immune response after a single dose of modified vaccinia Ankara-Bavaria Nordic vaccine against mpox in children: a national outbreak response. Lancet Infect Dis 2023;23(9):1042-50. [PMID: 37336224]
Laurenson-Schafer H., Sklenovska N., Hoxha A., et al. Description of the first global outbreak of mpox: an analysis of global surveillance data. Lancet Glob Health 2023;11(7):e1012-23. [PMID: 37349031]
Luong Nguyen L. B., Ghosn J., Durier C., et al. A prospective national cohort evaluating ring MVA vaccination as post-exposure prophylaxis for monkeypox. Nat Med 2022;28(10):1983-84. [PMID: 35831633]
Madewell Z. J., Charniga K., Masters N. B., et al. Serial interval and incubation period estimates of monkeypox virus infection in 12 jurisdictions, United States, May-August 2022. Emerg Infect Dis 2023;29(4):818-21. [PMID: 36863012]
Mailhe M., Beaumont A. L., Thy M., et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect 2023;29(2):233-39. [PMID: 36028090]
Mazzotta V., Cozzi-Lepri A., Lanini S., et al. Effect of tecovirimat on healing time and viral clearance by emulation of a target trial in patients hospitalized for mpox. J Med Virol 2023;95(6):e28868. [PMID: 37306318]
McLean J., Stoeckle K., Huang S., et al. Tecovirimat treatment of people with HIV during the 2022 mpox outbreak: a retrospective cohort study. Ann Intern Med 2023;176(5):642-48. [PMID: 37126820]
McQuiston J. H., Braden C. R., Bowen M. D., et al. The CDC domestic mpox response - United States, 2022-2023. MMWR Morb Mortal Wkly Rep 2023;72(20):547-52. [PMID: 37200231]
Mendoza R., Petras J. K., Jenkins P., et al. Monkeypox virus infection resulting from an occupational needlestick - Florida, 2022. MMWR Morb Mortal Wkly Rep 2022;71(42):1348-49. [PMID: 36264845]
Miura F., Backer J. A., van Rijckevorsel G., et al. Time scales of human mpox transmission in the Netherlands. J Infect Dis 2023;229(3):800-804. [PMID: 37014716]
Mizushima D., Shintani Y., Takano M., et al. Prevalence of asymptomatic mpox among men who have sex with men, Japan, January-March 2023. Emerg Infect Dis 2023;29(9):1872-76. [PMID: 37506678]
Montero Morales L., Barbas Del Buey J. F., Alonso Garcia M., et al. Post-exposure vaccine effectiveness and contact management in the mpox outbreak, Madrid, Spain, May to August 2022. Euro Surveill 2023;28(24):2200883. [PMID: 37318762]
Moschese D., Rizzo A., Raccagni A. R., et al. Plausibility of sexual behavior changes and role of vaccination in mpox outbreak control among MSM. Abstract 426. CROI; 2024 Mar 3-6; Denver, CO. https://www.croiconference.org/abstract/plausibility-of-sexual-behavior-changes-and-role-of-vaccination-in-mpox-outbreak-control-among-msm/
O'Laughlin K., Tobolowsky F. A., Elmor R., et al. Clinical use of tecovirimat (Tpoxx) for treatment of monkeypox under an investigational new drug protocol - United States, May-August 2022. MMWR Morb Mortal Wkly Rep 2022;71(37):1190-95. [PMID: 36107794]
Ogoina D., Iroezindu M., James H. I., et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis 2020;71(8):e210-14. [PMID: 32052029]
Overton E. T., Lawrence S. J., Stapleton J. T., et al. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020;38(11):2600-2607. [PMID: 32057574]
Palich R., Burrel S., Monsel G., et al. Viral loads in clinical samples of men with monkeypox virus infection: a French case series. Lancet Infect Dis 2023;23(1):74-80. [PMID: 36183707]
Paredes M. I., Ahmed N., Figgins M., et al. Underdetected dispersal and extensive local transmission drove the 2022 mpox epidemic. Cell 2024;187(6):1374-1386.e13. [PMID: 38428425]
Pepose J. S., Margolis T. P., LaRussa P., et al. Ocular complications of smallpox vaccination. Am J Ophthalmol 2003;136(2):343-52. [PMID: 12888060]
Perzia B., Theotoka D., Li K., et al. Treatment of ocular-involving monkeypox virus with topical trifluridine and oral tecovirimat in the 2022 monkeypox virus outbreak. Am J Ophthalmol Case Rep 2023;29:101779. [PMID: 36573234]
Philpott D., Hughes C. M., Alroy K. A., et al. Epidemiologic and clinical characteristics of monkeypox cases - United States, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71(32):1018-22. [PMID: 35951487]
Pollock E. D., Clay P. A., Keen A., et al. Potential for recurrent mpox outbreaks among gay, bisexual, and other men - United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72(21):568-73. [PMID: 37227964]
Quenelle D. C., Prichard M. N., Keith K. A., et al. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother 2007;51(11):4118-24. [PMID: 17724153]
Rao A. K., Petersen B. W., Whitehill F., et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71(22):734-42. [PMID: 35653347]
Rao A. K., Schrodt C. A., Minhaj F. S., et al. Interim clinical treatment considerations for severe manifestations of mpox - United States, February 2023. MMWR Morb Mortal Wkly Rep 2023;72(9):232-43. [PMID: 36862595]
Riser A. P., Hanley A., Cima M., et al. Epidemiologic and clinical features of mpox-associated deaths - United States, May 10, 2022-March 7, 2023. MMWR Morb Mortal Wkly Rep 2023;72(15):404-10. [PMID: 37053126]
Rosen J. B., Arciuolo R. J., Pathela P., et al. JYNNEOS effectiveness as post-exposure prophylaxis against mpox: challenges using real-world outbreak data. Vaccine 2024;42(3):548-55. [PMID: 38218669]
Rosenberg E. S., Dorabawila V., Hart-Malloy R., et al. Effectiveness of JYNNEOS vaccine against diagnosed mpox infection - New York, 2022. MMWR Morb Mortal Wkly Rep 2023;72(20):559-63. [PMID: 37339074]
Shah J., Saak T. M., Desai A. N., et al. Otolaryngologic manifestations among mpox patients: a systematic review and meta-analysis. Am J Otolaryngol 2023;44(6):103991. [PMID: 37487464]
Suner C., Ubals M., Tarin-Vicente E. J., et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect Dis 2023;23(4):445-53. [PMID: 36521505]
Tarin-Vicente E. J., Alemany A., Agud-Dios M., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet 2022;400(10353):661-69. [PMID: 35952705]
Thet A. K., Kelly P. J., Kasule S. N., et al. The use of vaccinia immune globulin in the treatment of severe mpox. virus infection in human immunodeficiency virus/AIDS. Clin Infect Dis 2023;76(9):1671-73. [PMID: 36571287]
Thornhill J. P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med 2022;387(8):679-91. [PMID: 35866746]
Titanji B. K., Tegomoh B., Nematollahi S., et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis 2022;9(7):ofac310. [PMID: 35891689]
Vaughan A. M., Cenciarelli O., Colombe S., et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European region, 7 March to 23 August 2022. Euro Surveill 2022;27(36):2200620. [PMID: 36082686]
Wolff Sagy Y., Zucker R., Hammerman A., et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat Med 2023;29(3):748-52. [PMID: 36720271]
Zachary K. C., Shenoy E. S. Monkeypox transmission following exposure in healthcare facilities in nonendemic settings: low risk but limited literature. Infect Control Hosp Epidemiol 2022;43(7):920-24. [PMID: 35676244]
Zhang X. S., Mandal S., Mohammed H., et al. Transmission dynamics and effect of control measures on the 2022 outbreak of mpox among gay, bisexual, and other men who have sex with men in England: a mathematical modelling study. Lancet Infect Dis 2024;24(1):65-74. [PMID: 37708908]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | May 06, 2024 |
| Intended users | Primary care providers and other clinicians in New York State who provide ambulatory care to individuals with or at risk of acquiring mpox disease |
| Lead author(s) |
Jacob R. McLean, DO; Jason E. Zucker, MD |
| Writing group |
Rona M. Vail, MD, AAHIVS; Sanjiv S. Shah, MD, MPH, AAHIVM, AAHIVS; Steven M. Fine, MD, PhD; Joseph P. McGowan, MD, FACP, FIDSA, AAHIVS; Samuel T. Merrick, MD, FIDSA; Asa E. Radix, MD, MPH, PhD, FACP, AAHIVS; Jessica Rodrigues, MPH, MS; Christopher J. Hoffmann, MD, MPH, MSc, FACP; Brianna L. Norton, DO, MPH; Charles J. Gonzalez, MD |
| Author and writing group conflict of interest disclosures | There are no author or writing group conflict of interest disclosures |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines | |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on May 13, 2024

