Purpose of This Guideline
Date of current publication: May 19, 2022
Lead author: Benjamin W. Tsoi, MD, MPH
Contributor: Monica Parker, PhD
Writing group: Steven M. Fine, MD, PhD; Joseph P. McGowan, MD, FACP, FIDSA; Rona Vail, MD; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Charles J. Gonzalez, MD; Christopher J. Hoffmann, MD, MPH
Committee: Medical Care Criteria Committee
Date of original publication: October 3, 2018
This guideline was developed by the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program to accomplish the following goals:
- Provide clinicians in NYS with up-to-date information on HIV testing policies and practices.
- Ensure awareness of and access to the standard 3-step HIV testing algorithm recommended by the Centers for Disease Control and Prevention (CDC) and the NYSDOH AI.
- Increase HIV testing in NYS to increase the number of people who know their HIV status.
- Ensure that clinicians recognize and respond to HIV testing as a gateway to care, such that an HIV diagnosis prompts a referral for HIV treatment and a negative HIV test result prompts a referral for HIV prevention services, including pre- and post-exposure prophylaxis (PrEP and PEP).
- Emphasize that rapid antiretroviral therapy (ART) initiation is the standard of care for all individuals diagnosed with HIV.
- Provide clinicians with information about the Wadsworth Center Bloodborne Viruses Laboratory services.
Accurate diagnosis or exclusion of HIV: This guideline provides an overview of the screening and diagnostic methods that are critical to accurate diagnosis or exclusion of HIV based on the CDC 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens and the Association of Public Health Suggested reporting language for the HIV laboratory diagnostic testing algorithm (see Figure 2: HIV Laboratory Testing Algorithm) APHL 2019; CDC 2018; CDC 2014.
Widespread use of the HIV antigen (Ag)/antibody (Ab) immunoassay (formerly known as the “4th-generation” test) can increase the number of people aware of their HIV status, including those who may transmit HIV during acute infection. The 2018 CDC algorithm testing sequence for detecting HIV Ags, Abs, and nucleic acids differs from previous HIV testing recommendations based on Ab screening followed by Western blot confirmation CDC 2018. The updated algorithm features a specific sequence of tests to provide maximal sensitivity, specificity, and accuracy for HIV detection.
Rapid ART: The current standard of care for a newly diagnosed person is same-day ART initiation. If an individual cannot start ART on the day of diagnosis, then every effort should be made to initiate ART as soon as possible and no later than 30 days after diagnosis.
New York State Law and Testing Requirements
| New York State Law |
|
Accessible and routine HIV testing for all individuals ≥13 years old is intended to expand the number of people who know their HIV status and facilitate entry into the continuum of care or prevention. NYS public health law requires clinicians to offer HIV testing to all patients ≥13 years old who receive care in hospital or primary care settings. Performing an HIV test for all patients ≥13 years old is a critical clinical and public health intervention for people with or at risk of acquiring HIV.
HIV testing is not an isolated activity; it is the entry point to the continuum of care and prevention. When an HIV test result is reactive, NYS law specifies that the healthcare provider who ordered testing (or their representative) is responsible for providing or arranging immediate follow-up HIV care. A negative HIV screening test result affords a critical opportunity to assess whether routine prevention education, including information about post-exposure prophylaxis (PEP), or a referral for HIV pre-exposure prophylaxis (PrEP) are indicated.
Consent: In November 2016, amendments to NYS public health law removed the requirement for written or oral informed consent before an HIV test is ordered; see HIV Testing, Reporting and Confidentiality in New York State 2017-18 Update: Fact Sheet and Frequently Asked Questions.
Box 1, below, provides an overview of HIV testing requirements in NYS. See also: NYS Expanded HIV Testing.
| Abbreviations: PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis. | |
| Box 1: New York State (NYS) Public Health Law HIV Testing and Reporting Requirements | |
| Who to test | NYS law mandates that physicians offer an HIV test to all patients ≥13 years old (or younger with risk) if a previous test is not documented, even in the absence of symptoms consistent with acute HIV. For more information, see NYSDOH HIV Testing. |
| Consent | HIV testing remains voluntary, and patients have the right to refuse an HIV test, but obtaining written or oral consent for testing is no longer required in any setting. At a minimum, patients must be advised verbally that an HIV test is going to be performed. |
| Minor consent | Minors may consent to their own HIV testing, treatment, and/or prevention services (such as PrEP and PEP) without parent/guardian involvement. |
| Pre-test counseling | Before HIV testing is performed, information about HIV must be provided verbally, in writing, through signage, or in any other patient-friendly audio-visual format. Placing a NYSDOH HIV testing clinic poster in a visible location or providing patients with the NYSDOH patient brochure on HIV testing are easy and convenient ways to provide patients with this necessary information. |
| Post-test counseling |
|
| Testing in pregnancy | HIV testing should be offered to pregnant individuals as early as possible during pregnancy and again during the third trimester for those who previously tested negative. |
| Reporting requirements |
|
| Partner services | Clinicians must explain to all individuals with a new diagnosis of HIV the importance of notifying any sex or needle-sharing partners. Throughout the notification process, names or personal identifiers, including the dates of exposure, are never revealed to partners. The anonymity and privacy of the original patient is the highest priority. For more information, see NYSDOH Information on Partner Services. |
| Nomenclature | In NYS, the terms “clinical/symptomatic HIV illness or AIDS,” “AIDS or HIV-related illness,” and other similar terms shall mean laboratory-confirmed HIV diagnosis (source: NYSDOH June 2016 Policy Statement: Defining Program Eligibility by HIV Status). |
| Resources |
|
Time to HIV Detection
Establishing the exact window period for an initial HIV test is challenging because the precise time of exposure is rarely known. Among individuals on PrEP who have breakthrough infections, the exact window period may be even more difficult to define due to partial suppression of viral replication and possible blunted or delayed immune response. PEP may also alter the time to detection of HIV.
Eclipse period refers to the time following an HIV exposure during which no available HIV test can detect the virus (see Figure 1, below). The duration of the eclipse period varies depending on characteristics of the infecting virus and the person infected Fiebig, et al. 2003. A study that applied modeling methods to estimate the time from exposure with subsequent infection to HIV RNA detection for people not receiving PrEP reported the median length of the eclipse period as 11.5 days Delaney, et al. 2017.
Window period refers to the period between an HIV exposure and when a test can detect HIV. The duration varies by person, test, and use of antiretrovirals (ARVs) as PrEP or PEP. It starts at the earliest time a test can accurately detect HIV and ends when the test consistently detects the biomarker quantified by the test. The antigen (Ag)/antibody (Ab) immunoassays detect HIV-1 and HIV-2 Abs and HIV-1 p24 Ag, which is present during acute HIV before Ab seroconversion (Ab production).
PrEP use can delay time from infection to seroconversion Spinelli, et al. 2021; Lee, et al. 2020. Very early treatment of acute HIV infection can, uncommonly, lead to delayed seroconversion or even seroreversion Stekler 2022; Hare, et al. 2006; Kassutto, et al. 2005.
Figure 1: HIV Window of Detection [a,b]
![Figure 1: HIV Window of Detection [a,b]](https://www.hivguidelines.org/wp-content/uploads/2023/07/NYSDOH-AI-HIV-Testing-Figure-1_7-20-2023_HG.jpg)
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; NAT, nucleic acid test.
Notes:
- Figure reproduced from CDC: HIV Diagnostic Tests.
- Without PrEP or PEP exposure; PrEP or PEP exposure may delay seroconversion. Very early treatment of acute HIV infection may also alter the serologic response Stekler 2022; Hare, et al. 2006; Kassutto, et al. 2005.
HIV Testing With the Standard 3-Step Algorithm
| RECOMMENDATIONS |
Step 1: HIV-1/2 Antigen/Antibody Immunoassay
Step 2: HIV-1/HIV-2 Antibody Differentiation Immunoassay
Step 3: HIV-1 Nucleic Acid Testing (qualitative or quantitative HIV RNA testing)
|
Abbreviations: Ab, antibody; Ag, antigen; ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CEI, Clinical Education Initiative; DHHS, U.S. Department of Health and Human Services; FDA, U.S. Food and Drug Administration; NAT, nucleic acid testing; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis. |
HIV-1/2 Antigen/Antibody Immunoassay (Step 1)
In 2018, the CDC issued a revised 3-step HIV testing algorithm for HIV diagnostic testing performed on serum and plasma specimens CDC 2018; CDC 2014. Advances in immunoassay technology have improved the sensitivity and specificity of HIV screening and diagnostic tests, which detect specific infection markers that may be virologic (viral proteins or nucleic acids) or immunologic (Abs produced in response to HIV infection). When requesting HIV diagnostic testing of anyone ≥2 years old, testing should be ordered from a laboratory that offers an FDA-approved HIV Ag/Ab immunoassay as an initial HIV test CDC 2018; CDC 2014. If the initial Ag/Ab immunoassay is reactive, the laboratory will usually follow the recommended algorithm steps to confirm or exclude laboratory evidence of HIV (see Appendix: HIV Immunoassays Available in New York State).
Figure 2, below, represents the 3-step standard HIV testing algorithm recommended by this committee and the CDC CDC 2018. Box 2, below, describes the testing services and assistance available through the NYSDOH Wadsworth Center.
Figure 2: HIV Laboratory Testing Algorithm [a]
![Figure 2: HIV Laboratory Testing Algorithm [a]](https://www.hivguidelines.org/wp-content/uploads/2022/12/NYSDOH-AI-HIV-Testing-Figure-2_11-1-2023_HG-768x656.jpg)
Abbreviations: Ab, antibody; Ag, antigen; APHL, Association of Public Health Laboratories; CDC, Centers for Disease Control and Prevention; ind, indeterminate; FDA, U.S. Food and Drug Administration; NAT, nucleic acid test; NYSDOH, New York State Department of Health; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis.
Notes:
- Adapted from CDC 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens and APHL Suggested reporting language for the HIV laboratory diagnostic testing algorithm.
- APHL and CDC continue to recommend that laboratories use an FDA-approved instrumented HIV-1/HIV-2 Ag/Ab immunoassay as the initial assay in the laboratory HIV testing algorithm for serum or plasma due to their superior sensitivity for detecting acute HIV infection. However, the FDA-approved single-use rapid HIV-1/HIV-2 Ag/Ab immunoassay may be used as the initial assay in the laboratory HIV testing algorithm for serum or plasma if an instrumented assay is not available.
- Become familiar with the laboratory’s internal testing algorithm and results-reporting policies. Many labs will reflex additional screening steps (such as HIV Ab differentiation immunoassay and HIV RNA) on the original sample without supplemental orders. Other labs may require additional samples or supplemental orders to complete all steps in the algorithm.
- This includes specimens reported as HIV-2 positive with HIV-1 cross-reactivity.
- Further testing may be performed to determine type.
- Per the Geenius package insert, specimens with this final assay interpretation should be retested with a new cartridge. If the final assay interpretation is again HIV-2 indeterminate, it should be reported as such and followed with an HIV-1 NAT.
- Most laboratories reflex directly to an HIV-1 RNA test without requiring an additional test order or new specimen, either by performing the test in-house or referring the specimen to another laboratory. If the laboratory is unable to or does not automatically reflex directly to the RNA test, clinicians should order an HIV-1 RNA test as soon as possible. To reflex directly to an HIV-1 RNA test, a test kit approved by either the FDA or NYSDOH to aid in diagnosing HIV-1 infection is required. If HIV-1 RNA is detected, acute HIV-1 is present, and clinicians should proceed with clinical evaluation. If no HIV-1 RNA is detected, the initial immunoassay result is presumed false positive.
- A negative HIV-1 NAT result and repeatedly HIV-2 indeterminate or HIV indeterminate Ab differentiation immunoassay result should be referred for testing with a different validated supplemental HIV-2 test (antibody test or NAT) if available. Alternatively, redraw and repeat algorithm in 2 to 4 weeks to assess HIV-2 infection.
Download figure: HIV Laboratory Testing Algorithm
| Abbreviations: Ab, antibody; Ag, antigen; ART, antiretroviral therapy; NAT, nucleic acid testing; RT-PCR, reverse transcription polymerase chain reaction. |
| Box 2: HIV Testing Services and Assistance Available Through the NYSDOH Wadsworth Center |
HIV-1/HIV-2 Diagnostic Testing (Phone: 518-474-2163)
HIV Testing for Newborns (Phone: 518-486-9605)
HIV-2 Viral Load Testing (Phone: 518-473-6007)
|
Screening for acute or established infection: In following the standard HIV laboratory testing algorithm, laboratories should perform initial HIV testing with an FDA-approved Ag/Ab immunoassay that detects HIV-1 and HIV-2 antibodies and HIV-1 p24 antigen. Initial testing screens for established HIV-1 or HIV-2 infection and acute HIV-1 infection. Confirming the presence of Abs to HIV-1 and HIV-2 identifies the majority of new HIV infections.
Results and next steps: A nonreactive initial Ag/Ab immunoassay is a negative HIV test result when used during routine HIV screening. If the initial test result is reactive, then supplemental testing with an HIV-1/HIV-2 Ab differentiation immunoassay is performed to rule out a false positive result. When the standard HIV laboratory testing algorithm is followed, laboratory reporting may include the initial HIV test result and supplemental testing results if it is reactive. A single test for establishing a laboratory diagnosis of HIV infection is not recommended; interpreting the complete set of results from a specific sequence of initial and supplemental tests is now recommended to establish laboratory evidence of HIV. Full results confirm reactivity and include an HIV-1/2 Ab differentiation immunoassay that detects and discriminates between HIV-1 and HIV-2 Abs with high specificity. If the supplemental antibody test (step 2) is negative or indeterminate, an HIV-1 RNA NAT (step 3) can verify acute HIV-1 infection (see Box 3, below).
HIV Ag/Ab immunoassay advantage: HIV-1/2 Ag/Ab immunoassays, formerly known as “4th-generation” immunoassays, detect both immunoglobulin G and M antibodies to HIV-1 and HIV-2 plus HIV-1 p24 antigen. These Ag/Ab immunoassays have a distinct advantage over screening tests that detect only Abs. Although these immunoassays cannot detect HIV during the eclipse period, when neither Ag nor RNA is detectable, the ability to identify both HIV-1 p24 Ag and HIV-1/2 Abs in a single screening test enables detection of HIV early in the acute period and throughout established infection, including in HIV controllers (see Table A.1: FDA-Approved HIV-1/2 Ag/Ab Immunoassays for Step 1 of the CDC Recommended Laboratory HIV Testing Algorithm for Serum or Plasma Specimens).
| KEY POINTS |
|
Available HIV Ag/Ab immunoassays: As of February 11, 2022, 7 FDA-approved HIV Ag/Ab immunoassays are available; all are approved for use in step 1 of the recommended HIV laboratory testing algorithm (see CDC: Advantages and Disadvantages of FDA-Approved HIV Assays Used for Screening). The Abbott Determine HIV-1/2 Ag/Ab Combo is the only FDA-approved HIV-1/2 Ag/Ab immunoassay rapid screening test. The CDC states that laboratory HIV-1/2 Ag/Ab immunoassays are preferred for use in step 1 of the algorithm due to their superior sensitivity for detecting HIV during acute infection. The Abbott Determine HIV-1/2 Ag/Ab Combo may be used with serum or plasma (not fingerstick whole blood) in this step when laboratory testing is not feasible CDC 2017.
Although Ag/Ab immunoassays are recommended for laboratories performing HIV testing, some laboratories may still use less sensitive assays for HIV screening. If an Ag/Ab immunoassay is not available, a laboratory-based HIV Ab immunoassay (3rd generation) test may be used. HIV Ab differentiation immunoassay offers the next best sensitivity for early detection; however, early acute HIV-1 infections may not be detected by an Ab differentiation immunoassay and it is only approved for supplemental testing to differentiate HIV-1/2 CDC 2018; CDC 2014. Several studies have shown that the HIV-1/2 Ab differentiation immunoassay and HIV-1 RNA test (NAT) performed according to the recommended laboratory algorithm are better than the Western blot for confirming a reactive HIV Ab differentiation immunoassay result Nasrullah, et al. 2013; Delaney, et al. 2011; Styer, et al. 2011; Wesolowski, et al. 2011.
Confirming a rapid screening test result: A reactive result to a rapid screening test should be given to the patient as soon as possible. Rapid screening tests may produce false positive results, particularly in populations not at high risk of HIV infection, and supplemental testing must be performed to confirm a reactive screening result.
If a rapid HIV screening test was performed on oral fluid or fingerstick whole blood, a blood specimen should be collected by venipuncture and handled according to the laboratory’s instructions.
The CDC advises laboratories to use the recommended HIV diagnostic testing algorithm to confirm all reactive rapid screening test results, including the Abbott Determine HIV-1/2 Combo rapid test when conducted on whole blood. An exception may be made when serum or plasma is screened with the Abbott Determine.
If reactive, the specimen may be tested directly with the supplemental HIV Ab differentiation immunoassay. The laboratory should test the confirmatory specimen with an HIV-1/2 Ag/Ab immunoassay approved for step 1 of the HIV diagnostic algorithm. If the specimen is not reactive, the rapid screening test result is interpreted as false positive, and no further testing is needed. If the immunoassay is reactive, specimen testing should continue according to the algorithm.
Collecting a tube of blood following a reactive rapid screening test may not always be possible or practical; supplemental testing of alternative specimen types may be necessary. The NYSDOH Wadsworth Center offers confirmatory testing of dried blood spots using an alternative algorithm for enrolled community-based HIV screening sites unable to collect venous blood.
| Abbreviations: Ab, antibody; Ag, antigen; HCV, hepatitis C virus; IgG, immunoglobulin G; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis.
Note:
|
||
| Box 3: Reasons for False Positive, False Negative, or Indeterminate HIV Test Results [a] | ||
| False Positive Results | False Negative Results | Comments |
|
|
|
HIV-1/HIV-2 Antibody Differentiation Immunoassay (Step 2)
Specimens with a reactive Ag/Ab immunoassay result (or repeatedly reactive, if repeat testing is recommended by the manufacturer or required by regulatory authorities) should be tested with an FDA-approved supplemental immunoassay that differentiates HIV-1 Abs from HIV-2 Abs. If the initial Ag/Ab immunoassay is reactive and the HIV-1/2 Ab differentiation immunoassay is positive for HIV-1 Abs, HIV-2 Abs, or HIV Abs, this should be interpreted as positive for HIV. If the specimen is positive for HIV Abs but cannot be differentiated as HIV-1 or HIV-2, clinicians should evaluate for HIV and contact the NYSDOH Wadsworth Center to obtain HIV-1 and HIV-2 RNA testing.
Geenius HIV 1/2 supplemental assay: On October 24, 2014, the Geenius HIV 1/2 Supplemental Assay (Bio-Rad Laboratories) received FDA approval for the confirmation and differentiation of individual HIV-1 and HIV-2 Abs. It is currently the only FDA-approved test for use in step 2 of the standard HIV laboratory testing algorithm.
This single-use immunochromatographic assay can confirm and differentiate individual HIV-1 and HIV-2 Abs in specimens found to be reactive by initial diagnostic screening. It is approved for use with whole blood, serum, or plasma specimens. Geenius uses 4 HIV-1 Ags derived from the core (p24), polymerase (p31), and envelope (gp41, gp160) proteins and 2 HIV-2 envelope Ags (gp36 and gp140). The test produces results within 30 minutes, and results must be read with the Geenius Reader system, which uses validated software to interpret the test results. The Geenius Reader can electronically transmit results to the laboratory’s information system, eliminating subjective result interpretation and error-prone manual data transcription.
If the final assay interpretation of the Geenius HIV 1/2 Supplemental Assay is “HIV-1 Positive,” “HIV-2 Positive,” or “HIV Positive,” then Abs are considered confirmed (see the package insert for additional information on the results reported in the final assay interpretation). The Geenius Reader may also produce a final assay interpretation that is indeterminate for either HIV-1, HIV-2, or untypable HIV.
If the test is nonreactive or indeterminate for any HIV type (HIV-1, HIV-2, or untypable HIV), the next step should be to test the specimens for HIV-1 RNA (qualitative or quantitative HIV RNA NAT), even if the result is HIV-2 indeterminate. Acute HIV-1 infection is much more common than HIV-2 infection, and nonspecific reactivity could cause an HIV-2 indeterminate result to occur in some cases. If HIV-1 RNA is not detected and the Geenius Reader result was HIV-2 indeterminate or HIV indeterminate, then an HIV-2 NAT may be warranted.
| KEY POINTS |
|
HIV-1 RNA Nucleic Acid Testing (Step 3)
If the HIV-1/2 Ab differentiation immunoassay is nonreactive or indeterminate, then HIV-1 RNA NAT (qualitative or quantitative) should be performed immediately to confirm or exclude HIV-1 infection.
For specimens that are reactive on the initial Ag/Ab immunoassay and nonreactive or indeterminate on the HIV-1/2 Ab differentiation immunoassay, the standard HIV laboratory testing algorithm recommends HIV RNA testing with an FDA-approved HIV-1 NAT, with results interpreted as follows:
- A reactive HIV-1 NAT result and nonreactive HIV-1/2 Ab differentiation immunoassay result indicates laboratory evidence for acute HIV-1 infection.
- A reactive HIV-1 NAT result and indeterminate HIV-1/2 Ab differentiation immunoassay result indicates the presence of HIV-1 infection confirmed by HIV-1 Abs.
- A negative HIV-1 NAT result and nonreactive or indeterminate HIV-1/2 Ab differentiation immunoassay result indicates a false positive result on the initial immunoassay.
Most laboratories reflex directly to an HIV-1 RNA test without requiring an additional test order or new specimen, either by performing the test in-house or referring the specimen to another laboratory. To reflex directly to an HIV-1 RNA test, a test kit approved by either the FDA or NYSDOH to aid in diagnosing HIV-1 infection is required. If HIV-1 RNA is detected, acute HIV-1 is present, and clinicians should proceed with clinical evaluation. If no HIV-1 RNA is detected, the initial immunoassay result is presumed false positive.
| KEY POINT |
|
Available HIV-1 RNA qualitative assay: Currently, the only NAT kit approved by the FDA for diagnostic use is the APTIMA HIV-1 RNA Qualitative Assay (Hologic Gen-Probe Inc). This NAT detects a specific region of the HIV-1 viral RNA genome by transcription-mediated amplification (TMA), a nucleic acid amplification method similar in principle to polymerase chain reaction (PCR). TMA differs from PCR in that the amplification occurs on a linear rather than logarithmic scale, and the amplification product is composed of single-stranded RNA rather than double-stranded DNA. TMA is FDA-approved for use with serum or plasma specimens and produces a qualitative result (i.e., “Detected” or “Not Detected”).
Data from analytical sensitivity studies presented in the package insert indicate that the APTIMA HIV-1 RNA Qualitative Assay achieved >98.5% detection for specimens containing 30 copies/mL of HIV-1 RNA and 100% detection for specimens containing 100 copies/mL. This detection level was also verified for HIV-1 specimen panels consisting of subtypes A, B, C, D, E, F, and G. The APTIMA HIV-1 RNA Qualitative Assay is performed by the NYSDOH and New York City Department of Health and Mental Hygiene public health laboratories and at several commercial laboratories; others are unable to support its use because of the expense and low volume of specimens that require qualitative RNA testing. Many laboratories already perform HIV-1 quantitative testing for viral load monitoring, and maintaining an additional qualitative test for diagnostic purposes may be impractical and not economically feasible.
The NYSDOH strongly recommends that all NYS birth facilities use the Pediatric HIV Testing Service at the Wadsworth Center. The Wadsworth Center uses the APTIMA HIV-1 RNA Qualitative Assay, which has been demonstrated to identify HIV earlier in non-breastfed infants than methods based on PCR amplification of proviral DNA.
Quantitative HIV-1 RNA tests: Quantitative HIV-1 RNA tests are widely available and approved by the FDA only to monitor the prognosis of HIV-1 infection and response to ART. Although regulatory restrictions may prevent laboratories from reflexing to a quantitative HIV-1 RNA test as part of the diagnostic testing algorithm, the NYSDOH recommends that clinicians order quantitative HIV-1 RNA for the presumptive diagnosis of acute HIV. With a quantitative HIV-1 RNA test, an HIV viral load ≥5,000 copies/mL is used to diagnose acute HIV when there is no antiretroviral exposure. A lower threshold of ≥200 copies/mL is appropriate for those receiving PrEP or PEP. The performance qualities of the HIV-1 viral load tests are discussed further in the NYSDOH AI guideline Virologic and Immunologic Monitoring in HIV Care.
For further guidance in the identification and management of acute HIV infection, see the NYSDOH AI guideline Diagnosis and Management of Acute HIV Infection.
| A NEW HIV DIAGNOSIS IS A CALL TO ACTION |
|
Diagnosis of HIV-2 Infection
| RECOMMENDATION |
Diagnosis of HIV-2 Infection
|
| Box 4: HIV-2 Related Services Available Through the Wadsworth Center |
|
The following services are available through the Wadsworth Center (phone: 518-474-2163):
|
|
Note: HIV-2 phenotypic and genotypic resistance testing is not offered at the Wadsworth Center or commercially available in the United States. |
HIV-2 antibodies (Abs) are confirmed by a reactive result to an HIV-1/2 antigen (Ag)/Ab immunoassay (step 1) and detection of HIV-2 Abs on a supplemental HIV-1/HIV-2 Ab differentiation assay (step 2). See the NYSDOH AI guideline Diagnosis and Management of HIV-2 in Adults for management recommendations.
Before the HIV-1/2 Ag/Ab and HIV-1/HIV-2 Ab differentiation immunoassays for HIV testing became widely available, clinicians suspected chronic HIV-2 infection in certain clinical scenarios, such as a declining CD4 cell count in an HIV-1–seropositive, untreated individual with an undetectable HIV-1 plasma viral load, or an opportunistic infection in an individual from West Africa who is not HIV-1 seropositive.
Currently, all HIV testing performed according to the Centers for Disease Control and Prevention (CDC) HIV testing algorithm begins with a U.S. Food and Drug Administration-approved HIV-1/2 Ag/Ab combination immunoassay CDC 2018, which detects HIV-1 p24 antigen and HIV-1 and HIV-2 Abs but not HIV-2 Ag. If the combination immunoassay is reactive, a supplemental HIV-1/HIV-2 Ab differentiation assay is performed. There are 4 scenarios, described below, in which clinicians should consider HIV-2 infection.
- HIV-1/HIV-2 differentiation assay is reactive for HIV-2 antibody: The individual is considered HIV-2 antibody positive, and a clinical evaluation for HIV-2 infection should be performed.
- HIV-1/HIV-2 differentiation assay is reactive for HIV-1 and HIV-2 antibody: The individual is considered HIV positive, undifferentiated, and HIV-1 RNA and HIV-2 RNA or DNA testing should be performed to confirm or exclude HIV-1/HIV-2 coinfection. A minority of individuals with HIV-2 are coinfected with HIV-1. Qualitative and quantitative HIV-2 viral load testing is available by contacting the Wadsworth Center (see Box 4, above).
- HIV-1/HIV-2 differentiation assay is nonreactive or indeterminate for HIV-1 and/or HIV-2 antibody: Plasma HIV-1 RNA testing should be performed to confirm or exclude acute HIV-1 infection CDC 2018.
- If the Ab differentiation assay is nonreactive or HIV-1 indeterminate and HIV-1 RNA is not detected, the individual is considered negative for HIV-1 and HIV-2.
- If the Ab differentiation assay is either HIV-2 indeterminate or HIV indeterminate and HIV-1 RNA is not detected, then HIV-2 RNA testing may be used if there is a suspicion for HIV-2 infection. However, because HIV-2 RNA levels can be low or undetectable in a person with HIV-2 infection, the absence of HIV-2 RNA does not exclude HIV-2 infection. Therefore, in a person at high risk for HIV-2 infection who has undetectable HIV-2 RNA, clinicians should consider testing for HIV-2 DNA or repeating the HIV testing algorithm in 2 to 4 weeks, starting with the HIV-1/2 Ag/Ab immunoassay. If results remain unclear, clinicians may consider obtaining other HIV-2–specific tests through public health or commercial laboratories or the CDC.
- Nonreactive HIV-1/2 Ag/Ab immunoassay and suspected recent exposure to HIV-2 (e.g., exposure from a sex partner from an HIV-2 endemic area): HIV-2 RNA testing may be required or the HIV testing algorithm may be repeated, beginning with the HIV-1/2 Ag/Ab immunoassay, 4 weeks (and not later than 12 weeks) after the first test.
Appendix: HIV Immunoassays Available in New York State
HIV screening and diagnostic tests are designed to detect specific infection markers, which may be virologic, such as viral proteins or nucleic acids, or immunologic, such as antibodies produced in response to HIV infection. HIV testing should begin with an immunoassay approved by the U.S. Food and Drug Administration (FDA) as an initial test to detect HIV-1 and HIV-2 infection. Advances in immunoassay technology have led to improved immunoassay sensitivity and/or specificity. See CDC: Advantages and Disadvantages of FDA-approved HIV Assays Used for Screening.
Clinical Laboratory Improvement Amendments (CLIA)-waived point-of-care HIV screening tests: The Centers for Medicare and Medicaid Services (CMS) regulates all laboratory testing in the United States performed on humans through the CLIA. The FDA categorizes tests that can use unprocessed specimens (whole blood or oral fluid), are easy to use, and have little risk of an incorrect result as “CLIA-waived” tests. These tests, which can be used at or near the patient care or nonclinical settings, are sometimes also used in laboratory settings and often provide results within 60 minutes (they are also referred to as “rapid tests”).
Laboratories licensed in New York State are exempt from CLIA. However, this exemption does not apply physicians’ office laboratories, which are required to have a CLIA certificate. In all other settings, NYS requirements meet or exceed CLIA requirements. In NYS, facilities that have to obtain a clinical laboratory permit must obtain a CLIA registration number from the Wadsworth Center Clinical Laboratory Evaluation Program (CLEP).
Rapid HIV screening tests: Rapid HIV screening tests can provide results within 60 minutes. Almost all rapid tests in the United States are CLIA-waived and can be used outside a laboratory. Rapid tests are an alternative option when HIV testing according to the standard algorithm is not possible or practical.
Laboratory HIV screening tests: Laboratory screening tests require serum or plasma specimens and are more complex than CLIA-waived tests. They require clinical laboratorians and instrumentation to perform the test or read results. Some laboratory screening tests provide results within 60 minutes.
In addition to improving sensitivity and specificity, several manufacturers have also developed HIV screening tests used with random access instrument systems. These systems eliminate or reduce the need for laboratories to batch samples and produce HIV screening test results very quickly, in many cases within 60 minutes. Immunoassays used for initial HIV screening can be divided into 2 categories:
- Enzyme or chemiluminescent immunoassays (EIAs, CIAs): Conducted by licensed technologists in a clinical laboratory.
- Rapid screening tests: Simple, single-use devices that produce a result in 30 minutes or less.
Interpreting screening results: All HIV immunoassays designed for initial screening may produce false positive results. Regardless of the test method, all reactive results should be interpreted as preliminary. Further testing is required to verify the reactive screening result and confirm the presence or absence of HIV infection.
| Abbreviations: Ab, antibody; Ag, antigen; CDC, Centers for Disease Control and Prevention; CMIA, chemiluminescent microparticle immunoassay; ECLIA, electrochemiluminescence immunoassay; EIA, enzyme immunoassay.
Note:
|
|
| Table A.1: FDA-Approved HIV-1/2 Ag/Ab Immunoassays for Step 1 of the CDC Recommended Laboratory HIV Testing Algorithm for Serum or Plasma Specimens | |
| Test | Method and Specimens |
|
|
|
|
|
|
|
|
|
|
|
|
Point-of-Care (Rapid) Screening Tests
The FDA has approved several HIV screening tests as CLIA-waived tests, which allows a test to be performed in non-laboratory, point-of-care (POC) settings. The FDA categorizes tests as CLIA-waived if they can use unprocessed specimens (whole blood or oral fluid), are easy to use, and have little risk of an incorrect result (see CDC: CLIA Certificate of Waiver). CLIA-waived tests are an option for initial HIV testing when it is not possible or practical to collect blood by venipuncture to submit to a clinical laboratory. These POC tests can produce results within 60 minutes and are usually also referred to as rapid tests. Currently, HIV rapid screening tests may be used only as initial screening tests; they are an alternative option when HIV testing according to the standard algorithm is not possible or practical.
HIV rapid screening tests are single-use test devices that produce results within 60 minutes but usually within 30 minutes. Table A.2, below, lists the characteristics of each of the 7 current FDA-approved rapid screening tests. Result interpretation is typically performed visually without instrumentation; the appearance of a line or circle in the appropriate area indicates a reactive result. The devices include a built-in procedural control that produces the expected appearance for a valid test result.
Although many rapid screening tests have been designed for POC use, clinical laboratories also use rapid screening tests for HIV screening when a result is needed very quickly or the laboratory’s overall testing volume is low. Depending on the device and its specific approval, laboratories may perform rapid screening tests using serum, plasma, or whole blood specimens collected by venipuncture. Each of the HIV rapid screening tests is restricted to the body fluid(s) that it was designed to analyze (see Table A.2, below). All CLIA-waived HIV screening tests may be used with plasma or serum specimens; however, laboratories processing these specimen types with CLIA-waived tests require a moderate- or high-complexity laboratory permit. The additional steps and instrumentation needed to process blood to plasma and serum add complexity to the test procedure; therefore, these tests are classified as moderate-complexity for serum and plasma specimens CDC 2016.
Rapid screening tests employ various technologies, and some devices are more sensitive for early detection than others. The Abbott Determine HIV-1/2 antigen/antibody (Ag/Ab) Combo test is distinguished by its ability to detect both HIV-1 p24 Ag and HIV-1 and HIV-2 Abs. Studies conducted by the CDC show that the Abbott Determine is capable of detecting HIV infection 1 to 2 weeks earlier than all other FDA-approved rapid screening tests but is less sensitive than the laboratory HIV-1/2 Ag/Ab combination immunoassays Masciotra, et al. 2013.
Aside from the Abbott Determine, all other FDA-approved rapid screening tests only detect HIV Abs and so are less sensitive for identifying acute HIV. These detect HIV Abs 6 to 12 days later than EIAs Masciotra, et al. 2013; Masciotra, et al. 2011.
| Abbreviations: Ab, antibody; Ag, antigen; CLIA, Clinical Laboratory Improvements Act; POC, point-of-care.
Notes:
|
||
| Table A.2: Characteristics of FDA-Approved Rapid HIV Tests | ||
| Test (manufacturer) | Sensitivity (95%) [a] | Specificity (95%) |
Chembio SURE CHECK HIV 1/2 Assay (Chembio Diagnostic Systems; package insert)
|
|
|
Chembio DPP HIV 1/2 Assay (Chembio Diagnostic Systems; package insert)
|
|
|
Chembio HIV 1/2 STAT-PAK Assay (Chembio Diagnostic Systems; package insert)
|
|
|
Abbott Determine HIV-1/2 Ag/Ab Combo (Abbott; package insert)
|
|
|
INSTI HIV-1/HIV-2 Antibody Test (bioLytical Laboratories; package insert)
|
|
|
OraQuick ADVANCE Rapid HIV-1/2 Antibody Test (OraSure Technologies; package insert)
|
|
|
Uni-Gold Recombigen HIV-1/2 (Trinity Biotech; package insert)
|
|
|
Home-Based Tests
The CDC encourages health departments to allow HIV self-testing (i.e., HIV testing not performed by trained professionals). Currently, only 2 in-home HIV tests are in use: Home Access HIV-1 Test System (Home Access Health) and OraQuick In-Home HIV Test (OraSure Technologies).
Home Access HIV-1 Test System: FDA-approved in 1996 for sale in the United States. The individual collects blood from a fingerstick and transfers the blood onto filter paper, which is mailed to a facility for analysis using FDA-approved tests to detect HIV-1 Abs. If a reactive result is obtained, a trained HIV counselor conducts post-test counseling by telephone. If a negative result is obtained, pre- and post-test counseling consists of a recorded message (a counselor is also available if requested). Results are available in either 3 or 7 days.
OraQuick In-Home HIV Test: FDA-approved in 2012 for over-the-counter sales for people ≥17 years old to use with oral fluid to obtain a result in 20 minutes. The user swipes an oral swab along the gums to collect a sample, which is inserted into a test tube provided in the kit. OraSure provides 24/7 support in English and Spanish by telephone for technical questions, interpretation of results, counseling, and referrals for follow-up support and care. For more information about HIV testing of oral specimens, see the guideline section Alternative HIV Tests: Oral and Urine Specimens, below.
When discussing the possible use of self-tests with patients, care providers should emphasize that although these tests are generally accurate Figueroa, et al. 2018, errors in specimen collection, processing or interpreting results may occur, and should also stress the importance of using only tests that are FDA-approved. The possibility of these types of errors vary between the Home Access HIV-1 fingerstick mail-in test and the OraQuick in-home test kit.
Alternative HIV Tests: Oral and Urine Specimens
A limited number of FDA-approved assays may be performed on body fluids other than blood for HIV diagnostic testing. Advantages to oral fluid and urine specimens include noninvasive sample collection in settings where phlebotomy is not available and reduced risk of occupational exposure to infectious agents. Disadvantages include reduced sensitivity and specificity of the test methods FDA(a) 2009; FDA(b) 2009.
Oral fluid specimens: Oral fluid is not saliva but oral mucosal transudate (OMT) obtained by swabbing the gums. Though Abs are detectable in OMT, they are present at concentrations 800- to 1,000-fold lower than those found in serum or plasma. The FDA has approved 1 EIA for use on oral fluid specimens. The Avioq HIV-1 Microelisa System may be performed on OMT specimens collected using the OraSure Oral Fluid Collection Device, and reactive results must be confirmed.
The OraQuick ADVANCE Rapid HIV-1/2 Antibody Test (OraSure Technologies) is the only rapid screening test that has been FDA-approved for use with oral fluid (see Table A.2, above). The test detects both HIV-1 and HIV-2 Abs but cannot distinguish between them. This test is CLIA-waived and may be used with oral fluid specimens in POC and nonclinical testing sites.
Urine specimens: HIV-1 Abs may be detected in urine. One screening test and one Western blot are FDA-approved for use with urine specimens. Although urine specimens are commonly used for HIV testing in particular situations, such as insurance company testing, HIV tests for urine specimens do not offer adequate sensitivity or specificity for general diagnostic use and should be avoided. Both nonreactive and reactive results of urine HIV testing should be confirmed with standard serological testing, preferably an HIV-1/2 Ag/Ab immunoassay.
Not Recommended for the HIV Diagnostic Laboratory Testing Algorithm
Notably, the Western blot has been eliminated from the recommended testing algorithm (see Figure 2: HIV Laboratory Testing Algorithm) and is used only in the situations described previously. The Western blot is very specific, and false positive results are rare, but it has several important disadvantages. The HIV-1 test is less sensitive than Ag/Ab immunoassays and will produce false negative or indeterminate results on specimens collected before or during seroconversion Masciotra, et al. 2011; Styer, et al. 2011; Owen, et al. 2008. It also produces indeterminate results for various other reasons and misclassifies the majority of HIV-2 infections Lasry, et al. 2014; Nasrullah, et al. 2011; Torian, et al. 2010.
The indirect immunofluorescence assay (IFA) is another supplemental test designed to confirm the presence of HIV-1 Abs. The IFA is not routinely performed for HIV diagnostic testing and is not recommended as a supplemental test for confirming the presence of HIV Abs.
All Recommendations
| ALL RECOMMENDATIONS: HIV TESTING |
Step 1: HIV-1/2 Antigen/Antibody Immunoassay
Step 2: HIV-1/HIV-2 Antibody Differentiation Immunoassay
Step 3: HIV-1 Nucleic Acid Testing (qualitative or quantitative HIV RNA testing)
Diagnosis of HIV-2 Infection
|
Abbreviations: Ab, antibody; Ag, antigen; ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CEI, Clinical Education Initiative; DHHS, U.S. Department of Health and Human Services; FDA, U.S. Food and Drug Administration; NAT, nucleic acid testing; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis. |
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
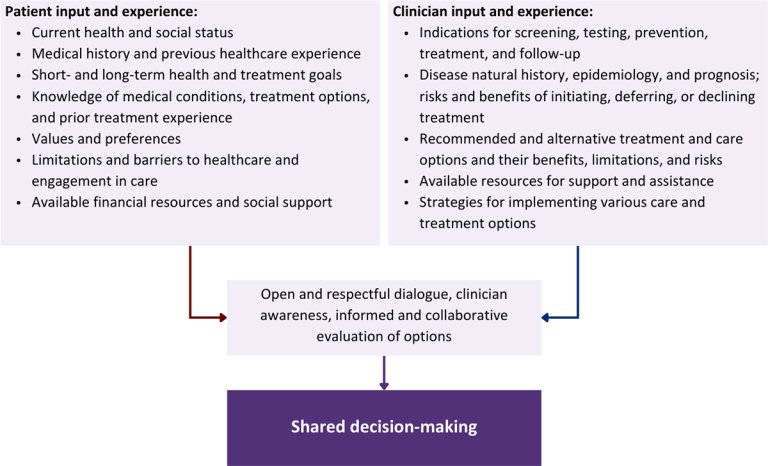
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
APHL. Suggested reporting language for the HIV laboratory diagnostic testing algorithm. 2019 Jan. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2019Jan-HIV-Lab-Test-Suggested-Reporting-Language.pdf [accessed 2021 Dec 8]
CDC. Laboratory testing for the diagnosis of HIV infection: updated recommendations. 2014 Jun 27. https://stacks.cdc.gov/view/cdc/23447 [accessed 2018 Mar 18]
CDC. Rapid HIV tests suitable for use in clinical settings (CLIA-moderate complexity). 2016 Aug 10. https://www.cdc.gov/hiv/pdf/testing/rapid-hiv-tests-clinical-moderate-complexity.pdf [accessed 2021 Nov 29]
CDC. Technical update: use of the Determine HIV 1/2 Ag/Ab combo test with serum or plasma in the laboratory algorithm for HIV diagnosis. 2017 Oct 4. https://stacks.cdc.gov/view/cdc/48472 [accessed 2021 Dec 3]
CDC. 2018 quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. 2018 Jan. https://stacks.cdc.gov/view/cdc/50872 [accessed 2021 Nov 29]
Delaney K. P., Hanson D. L., Masciotra S., et al. Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clin Infect Dis 2017;64(1):53-59. [PMID: 27737954]
Delaney K. P., Heffelfinger J. D., Wesolowski L. G., et al. Performance of an alternative laboratory-based algorithm for HIV diagnosis in a high-risk population. J Clin Virol 2011;52 Suppl 1:S5-10. [PMID: 22019251]
FDA(a). Avioq HIV-1 Microelisa System. 2009 Aug. https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/UCM185273.pdf [accessed 2018 Mar 16]
FDA(b). OraQuick ADVANCE Rapid HIV-1/2 Antibody Test. 2009 Feb. https://www.fda.gov/media/73607/download [accessed 2018 Mar 16]
Fiebig E. W., Wright D. J., Rawal B. D., et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003;17(13):1871-79. [PMID: 12960819]
Figueroa C., Johnson C., Ford N., et al. Reliability of HIV rapid diagnostic tests for self-testing compared with testing by health-care workers: a systematic review and meta-analysis. Lancet HIV 2018;5(6):e277-90. [PMID: 29703707]
Hare C. B., Pappalardo B. L., Busch M. P., et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis 2006;42(5):700-708. [PMID: 16447118]
Kassutto S., Johnston M. N., Rosenberg E. S. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin Infect Dis 2005;40(6):868-73. [PMID: 15736021]
Lasry A., Sansom S. L., Wolitski R. J., et al. HIV sexual transmission risk among serodiscordant couples: assessing the effects of combining prevention strategies. AIDS 2014;28(10):1521-29. [PMID: 24804859]
Lee S. S., Anderson P. L., Kwan T. H., et al. Failure of pre-exposure prophylaxis with daily tenofovir/emtricitabine and the scenario of delayed HIV seroconversion. Int J Infect Dis 2020;94:41-43. [PMID: 32173577]
Masciotra S., Luo W., Youngpairoj A. S., et al. Performance of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol 2013;58 Suppl 1:e54-58. [PMID: 23911678]
Masciotra S., McDougal J. S., Feldman J., et al. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol 2011;52 Suppl 1:s17-22. [PMID: 21981983]
Nasrullah M., Ethridge S. F., Delaney K. P., et al. Comparison of alternative interpretive criteria for the HIV-1 Western blot and results of the Multispot HIV-1/HIV-2 Rapid Test for classifying HIV-1 and HIV-2 infections. J Clin Virol 2011;52 Suppl 1:s23-27. [PMID: 21993309]
Nasrullah M., Wesolowski L. G., Meyer W. A., et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS 2013;27(5):731-37. [PMID: 23135170]
Owen S. M., Yang C., Spira T., et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J Clin Microbiol 2008;46(5):1588-95. [PMID: 18322061]
Spinelli M. A., Lowery B., Shuford J. A., et al. Use of drug-level testing and single-genome sequencing to unravel a case of human immunodeficiency virus seroconversion on pre-exposure prophylaxis. Clin Infect Dis 2021;72(11):2025-28. [PMID: 32686825]
Stekler J. Seroconversion, seroreversion, and serowaffling among participants in Project DETECT. Advancing HIV, STI and Viral Hepatitis Testing Conference; 2022 Mar 31. https://hivtestingconference.org/wp-content/uploads/2022/04/Seckler_C1Session4.pdf
Styer L. M., Sullivan T. J., Parker M. M. Evaluation of an alternative supplemental testing strategy for HIV diagnosis by retrospective analysis of clinical HIV testing data. J Clin Virol 2011;52 Suppl 1:s35-40. [PMID: 22018662]
Torian L. V., Eavey J. J., Punsalang A. P., et al. HIV type 2 in New York City, 2000-2008. Clin Infect Dis 2010;51(11):1334-42. [PMID: 21039219]
Wesolowski L. G., Delaney K. P., Hart C., et al. Performance of an alternative laboratory-based algorithm for diagnosis of HIV infection utilizing a third generation immunoassay, a rapid HIV-1/HIV-2 differentiation test and a DNA or RNA-based nucleic acid amplification test in persons with established HIV-1 infection and blood donors. J Clin Virol 2011;52 Suppl 1:s45-49. [PMID: 21995934]
Updates, Authorship, and Related Resources
| Updates, Authorship, and Related Resources | |
| Date of original publication | October 03, 2018 |
| Date of current publication | May 19, 2022 |
| Highlights of changes, additions, and updates in the May 19, 2022 edition |
|
| Intended users | New York State care providers who do provide or should provide HIV testing to individuals who may be at risk of acquiring HIV or report a potential exposure |
| Lead author |
Benjamin W. Tsoi, MD, MPH |
| Contributing author |
Monica Parker, PhD Monica Parker, PhD; |
| Writing group |
Steven M. Fine, MD, PhD; Joseph P. McGowan, MD, FACP, FIDSA; Rona Vail, MD; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Charles J. Gonzalez, MD; Christopher J. Hoffmann, MD, MPH |
| Author and writing group conflict of interest disclosures |
Joseph P. McGowan, MD, FACP, FIDSA: Institutional Pharma grant recipient/support, clinical trial; Gilead |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI resources |
Guidelines
Podcast |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on July 17, 2025

