Purpose of This Guideline
Date of current publication: May 20, 2022
Lead author: Rona M. Vail, MD
Writing group: Steven M. Fine, MD, PhD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Jessica Rodrigues; Christopher J. Hoffmann, MD, MPH; Lyn C. Stevens, MS, NP, ACRN; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: October 10, 2019
HIV prevention with pre-exposure prophylaxis (PrEP) is the use of antiretroviral medications by individuals who do not have HIV to reduce their risk of acquiring HIV. PrEP is a cornerstone of HIV prevention and is strongly endorsed by New York State. However, it is underutilized, particularly by communities disproportionately affected by HIV.
Goals
The New York State Ending the Epidemic (ETE) initiative presents strategies from the ETE Task Force to decrease HIV prevalence and end the AIDS epidemic in New York State. The third pillar of the 3-pillar ETE plan is facilitating access to PrEP as a proven strategy to prevent HIV acquisition among individuals at risk. Its inclusion as a pillar of this initiative emphasizes the safety and effectiveness of PrEP as a method to prevent HIV infection. This guideline was developed by the Medical Care Criteria Committee of the NYSDOH AI Clinical Guidelines Program to provide clinicians throughout New York State with the recommendations needed to successfully start and continue patients on PrEP.
In support of the ETE initiative and to reduce new HIV infections in New York State, the goals of this guideline are to:
- Increase awareness and knowledge of PrEP efficacy among clinicians in New York State.
- Assist clinicians in identifying candidates for PrEP and increasing awareness of, access to, and uptake of PrEP among individuals in New York State at risk of acquiring HIV through sexual and drug use exposures.
- Discuss the barriers to PrEP access and encourage clinicians to assist PrEP candidates in reducing or eliminating these barriers.
- Provide clinicians with the information needed to help a PrEP candidate make the best choice regarding oral versus injectable PrEP and daily versus on-demand PrEP.
- Provide clinicians with evidence-based recommendations for PrEP initiation, management, monitoring, and discontinuation.
Note on “experienced” and “expert” HIV care providers: Throughout this guideline, when reference is made to “experienced HIV care provider” or “expert HIV care provider,” those terms are referring to the following 2017 NYSDOH AI definitions:
- Experienced HIV care provider: Practitioners who have been accorded HIV Experienced Provider status by the American Academy of HIV Medicine or have met the HIV Medicine Association’s definition of an experienced provider are eligible for designation as an HIV Experienced Provider in New York State. Nurse practitioners and licensed midwives who provide clinical care to individuals with HIV in collaboration with a physician may be considered HIV Experienced Providers as long as all other practice agreements are met (8 NYCRR 79-5:1; 10 NYCRR 85.36; 8 NYCRR 139-6900). Physician assistants who provide clinical care to individuals with HIV under the supervision of an HIV Specialist physician may also be considered HIV Experienced Providers (10 NYCRR 94.2)
- Expert HIV care provider: A provider with extensive experience in the management of complex patients with HIV.
PrEP Access and Coverage
U.S. Food and Drug Administration (FDA)-approved agents for PrEP: Tenofovir disoproxil fumarate 300 mg/emtricitabine 200 mg in a fixed-dose tablet (TDF/FTC; brand name Truvada) is approved by the FDA for use as PrEP as part of a comprehensive HIV prevention strategy for individuals at risk of acquiring HIV. Multiple randomized, placebo-controlled clinical trials have demonstrated the efficacy of TDF/FTC as PrEP for preventing HIV infection in all populations.
Tenofovir alafenamide 25 mg/emtricitabine 200 mg in a fixed-dose tablet (TAF/FTC; brand name Descovy) was found to be noninferior to TDF/FTC in a study of men who have sex with men (MSM) and a small number of transgender women who have sex with men Mayer, et al. 2020. TAF/FTC was approved by the FDA in October 2019 for HIV prevention through sexual exposure in those groups; it has not yet been approved for prevention of HIV through receptive vaginal exposure FDA(a) 2019.
Long-acting injectable cabotegravir (CAB LA; brand name Apretude), an integrase strand transfer inhibitor given as a bimonthly injection after a 5-week oral CAB (brand name Vocabria) lead-in and after 2 initial injections given 4 weeks apart, was statistically superior to oral TDF/FTC as PrEP in MSM and transgender women Landovitz, et al. 2021 and in cisgender women Delany-Moretlwe, et al. 2022 CAB LA was approved by the FDA in December 2021 for prevention of HIV via all sexual exposures FDA(b) 2021.
PrEP uptake: Although new HIV infections and diagnoses have steadily declined in New York State, these decreases have not been uniform across all groups. MSM and people of color, particularly young MSM and women of color, continue to be overrepresented among those newly infected and diagnosed with HIV NYSDOH 2018. Computer simulation modeling suggests that increased PrEP uptake will be the single largest contributor to further reductions in new HIV infections and key to ending the HIV epidemic in New York State NYSDOH 2018. However, data indicate that people of color, women, and individuals accessing Medicaid—3 groups overrepresented among people with HIV—are accessing PrEP at lower levels than other groups in whom the disease burden is high ETE Dashboard 2020. For example, in 2020, 19.4% of new HIV diagnoses in New York State were in women, but just 8.1% of all individuals who accessed PrEP in New York State were women. Nationally, although White MSM account for 30% of new HIV infections, nearly 75% of prescriptions for PrEP in the United States have gone to White MSM, illustrating the need to improve outreach to other communities affected by HIV Townes, et al. 2021; Jenness, et al. 2019; Goedel, et al. 2018; Goldstein, et al. 2018.
Barriers to PrEP access and use: The NYSDOH AI recognizes that a comprehensive approach is necessary to ensure that individuals who will most benefit from the use of PrEP have access to it and that their care is managed effectively while they are taking PrEP. This guideline addresses some structural barriers to PrEP care by advocating for individualization and flexibility in care-delivery models, including monitoring schedules and dosing options.
Barriers to PrEP access include:
- Suboptimal awareness or acceptance of PrEP among some individuals at risk for acquiring HIV and their care providers Townes, et al. 2021; Bazzi, et al. 2018; Mayer, et al. 2018; Rael, et al. 2018; Bien, et al. 2017; Blackstock, et al. 2017; King, et al. 2014
- Lack of retention in PrEP care due to individual and structural barriers D'Angelo, et al. 2021; Serota, et al. 2020; Chan, et al. 2016
- Stigma, which may keep people who would benefit from PrEP from using it
- Disparities in access to PrEP among populations at high risk of HIV acquisition, including MSM of color, transgender women, Black women, and people who inject drugs Biello, et al. 2018; CDC(a) 2018; Garnett, et al. 2018; Morgan, et al. 2018; Sullivan, et al. 2018; Lancki, et al. 2018; Page, et al. 2017; Philbin, et al. 2016; Sevelius, et al. 2016; King, et al. 2014. Emerging evidence suggests that transgender MSM are also at high risk for HIV acquisition Pitasi, et al. 2019; Scheim, et al. 2017 and are a population for whom PrEP outreach and access are needed.
The availability of injectable PrEP creates unique opportunities and potential challenges for systems to incorporate this option. Populations at the highest risk of acquiring HIV should be prioritized for PrEP outreach and access to ensure they are aware of PrEP and its benefits. PrEP programs will have to develop systems and protocols to make injectable PrEP available.
It is also crucial to address barriers and expand access to PrEP by increasing the number of medical care providers who are aware of and willing to prescribe PrEP. Care providers need to examine any unconscious biases that may influence their willingness to offer PrEP to patients Calabrese, et al. 2018; Edelman, et al. 2017; Calabrese, et al. 2014, avoid making assumptions about sexual practices, and develop comfort and facility in obtaining routine sexual histories and asking about injection drug use practices to identify potential PrEP candidates. Regardless of disclosed risk, if a patient asks for PrEP and it is not medically contraindicated, they should be offered a prescription and appropriate follow-up.
Coverage under the Affordable Care Act (ACA): In August 2023, the U.S. Preventive Services Task Force (USPSTF) published an updated grade A recommendation stating that clinicians should “prescribe preexposure prophylaxis using effective antiretroviral therapy to persons at increased risk of HIV acquisition to decrease the risk of acquiring HIV” USPSTF 2023. The updated USPSTF recommendation statement also includes a review of the evidence on newer HIV PrEP medications, including oral TAF/FTC and long-acting CAB, in addition to oral TDF/FTC. This federal recommendation recognizes PrEP as a preventive service to be covered under the ACA, a significant step toward increasing access to PrEP, and further affirms PrEP as a highly effective HIV prevention strategy that clinicians can and should provide to their patients.
Coverage in New York State: In July 2019, the New York State Department of Financial Services issued a Circular Letter instructing New York State insurers to cover PrEP without cost-sharing, including copays and deductibles, which have been a major financial barrier for many consumers.
| NYSDOH RESOURCES |
|
PrEP for Individuals at Risk of Acquiring HIV
| RECOMMENDATION |
Indications
Contraindications to PrEP
|
Abbreviations: ART, antiretroviral therapy; CrCl, creatinine clearance; nPEP, non-occupational post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TDF/FTC; tenofovir disoproxil fumarate/emtricitabine (brand name Truvada). |
PrEP Candidates
PrEP is part of a comprehensive HIV prevention plan and should be offered to individuals, including adolescents who meet the prescribing criteria, who are assessed or who self-identify as being at increased risk of acquiring HIV through sexual or injection drug exposure.
Box 1, below, shows candidates who should be offered PrEP (e.g., those at risk of acquiring HIV through rectal, genital, or blood exposures, and those who request PrEP) and factors that do not disqualify candidates from PrEP.
| Box 1: Candidates for PrEP |
Offer PrEP to individuals who are candidates for PrEP, including those who:
Do not withhold PrEP from eligible candidates who:
|
|
Abbreviations: nPEP, non-occupational post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection; U=U, undetectable=untransmittable. |
Men who have sex with men (MSM): Studies have demonstrated that TDF/FTC as PrEP is highly effective in MSM Mayer, et al. 2020; McCormack, et al. 2016; Molina, et al. 2015; Grant, et al. 2014; Grant, et al. 2010. Although initial data from the iPrEx trial demonstrated only a 44% reduction in the rate of HIV acquisition, there was a 92% reduction in sexual transmission when tenofovir was detectable in the blood. No seroconversions occurred in individuals with therapeutic plasma concentrations of TDF/FTC Grant, et al. 2010. In the PROUD study TDF/FTC as PrEP had an overall efficacy rate of 86%, and there were no HIV infections in participants who took TDF/FTC as prescribed McCormack, et al. 2016. Based on intracellular concentrations of tenofovir diphosphate, HIV risk-reduction efficacy in the iPrEX study was estimated to be 99% with 7 doses per week, 96% with 4 doses per week, and 76% with 2 doses per week Anderson, et al. 2012.
The DISCOVER study showed both TDF/FTC and TAF/FTC to be highly effective in reducing the risk of HIV infection in MSM, with a combined total of 22 participants with new HIV infections in 8,756 person-years of follow-up; all except 2 were either infected at baseline or had low tenofovir plasma concentrations at the time of infection Mayer, et al. 2020. In the HPTN 083 study, both TDF/FTC and long-acting injectable cabotegravir (CAB LA; brand name Apretude) prevented HIV in MSM and transgender women. However, CAB LA was statistically superior, with 14 incident infections among participants receiving the regimen, compared with 41 incident infections among participants taking TDF/FTC during the blinded phase of the study, mostly driven by adherence and low tenofovir levels in individuals seroconverting on TDF/FTC Landovitz, et al. 2022; Landovitz, et al. 2021.
Transgender women: In the iPrEx study, TDF/FTC as PrEP was effective in transgender women who adhered to the regimen. In a subanalysis of transgender women in the iPrEx study, there was no difference in rates of HIV infection between the PrEP and placebo groups; however, none of the transgender women who seroconverted had detectable tenofovir levels, and there were no HIV infections in those who had adequate tenofovir levels Deutsch, et al. 2015. Subsequent analysis has shown significant differences in the baseline characteristics between MSM and transgender women in the iPrEX study Mehrotra, et al. 2019.
Multiple studies have shown that TDF/FTC does not alter estrogen levels Grant, et al. 2021; Shieh, et al. 2019; Hiransuthikul, et al. 2019. Small studies have raised the possibility that estrogen lowers tenofovir levels by approximately 12% in the plasma of transgender women compared with cisgender men or lowers levels of active metabolite in rectal tissues Cottrell, et al. 2019; Shieh, et al. 2019, although the significance of this difference is unclear. In the larger iBrEATHe study, which used directly observed daily oral TDF/FTC as PrEP for 4 weeks in transgender women on consistent hormone therapy, differences in tenofovir levels measured by dried blood spots did not meet statistical significance Grant, et al. 2021. In the ImPrEPT study among transgender individuals taking TDF/FTC as PrEP, no interactions between hormone therapy and PrEP were reported Blumenthal, et al. 2022. It is possible that higher levels of adherence are needed among transgender women using estrogens and taking TDF/FTC for PrEP than among cisgender men, but levels achieved with daily dosing will confer protection from TDF/FTC as PrEP.
TAF/FTC was effective in transgender women in the DISCOVER study, but transgender women made up only 1% of the study population Mayer, et al. 2020. There are no formal pharmacokinetic studies of the effects of estrogen at doses used for gender-affirming care on TAF levels, but intracellular PBMC concentrations of tenofovir diphosphate were similar between transgender women taking gender-affirming hormone therapy and MSM in the DISCOVER study Cespedes, et al. 2020. There are also no pharmacokinetic studies of the effects of estrogen at doses used for gender-affirming therapy on CAB LA levels, but in the HPTN 083 study, transgender women made up 12.5% of the study population, and CAB LA was effective across all subpopulations. Additionally, oral CAB pharmacokinetics were not significantly altered by low-dose hormones used for oral contraceptives in the HPTN 077 study Blair, et al. 2020.
Transgender men: Data are lacking regarding the efficacy of TDF/FTC, TAF/FTC, and CAB LA as PrEP for transgender men who have sex with either cisgender men or transgender women, despite the increased risk of HIV acquisition in this population Pitasi, et al. 2019; Scheim, et al. 2017. In the iBrEATHe study, TDF/FTC did not lower testosterone levels in transgender men compared with controls, and testosterone did not lower tenofovir levels in transgender men compared with cisgender men Grant, et al. 2021. Tenofovir levels were lower in transgender men than in cisgender women; however, levels were still in a range consistent with effective PrEP with TDF/FTC in clinical trials. There are no data yet on whether testosterone therapy affects CAB levels. However, there is no specific reason to believe that CAB LA will be less effective for vaginal or anal exposures in preventing HIV infection in this population.
Cisgender men and women: Studies have demonstrated that TDF/FTC as PrEP is effective for cisgender men and women. In the Partners PrEP study, there was a 67% overall reduction in HIV acquisition in cisgender men and women and a 90% reduction in those with detectable drug in their blood Baeten, et al. 2012. In the TDF2 study, there was a 62% overall reduction in HIV acquisition, and there were only 2 seroconversions in participants who had detectable drug Thigpen, et al. 2012. However, the FEM-PrEP and VOICE trials did not demonstrate a benefit of TDF/FTC as PrEP for cisgender women, although subsequent analyses found that the lack of effect was associated with poor adherence to the prescribed daily PrEP regimen Van Damme, et al. 2012. TAF/FTC has not been studied in cisgender women. CAB LA was found to be statistically superior to TDF/FTC in the HPTN 084 study of cisgender women, in which there were 34 incident infections in the TDF/FTC arm but only 4 incident infections in the CAB LA arm, a difference driven by adherence to oral medication Delany-Moretlwe, et al. 2022.
HIV-serodifferent couples: PrEP may be useful for individuals in a serodifferent partnership, even if the partner with HIV is taking suppressive ART. Data from the Partners in Prevention HSV/HIV Transmission Study and the HPTN 052 study demonstrated reductions up to 92% and 96%, respectively, in HIV transmission risk in serodifferent heterosexual couples when the partner with HIV was on suppressive ART Cohen, et al. 2011. HIV is transmissible if an individual’s viral load is not fully suppressed, which may take up to 6 months or longer after ART initiation. Once an undetectable viral load is achieved and maintained, HIV is not sexually transmissible Mujugira, et al. 2016. For more information, see the NYSDOH AI U=U Guidance for Implementation in Clinical Settings.
In the Partners Demonstration Project, use of TDF/FTC as PrEP as a “bridge” was highly effective in protecting the individual without HIV during the first 6 months of a partner’s ART. Subsequently, in the Partner, Partner 2, and Opposites Attract studies, there were no sexual transmissions between serodifferent partners when the partner with HIV had an undetectable viral load Baeten, et al. 2016. In September 2017, the NYSDOH endorsed the undetectable = untransmittable (U=U) consensus statement from the Prevention Access Campaign Prevention Access Campaign 2019; NYSDOH 2017.
In a serodifferent partnership, the partner who does not have HIV may decide to use PrEP even if the partner with HIV has achieved an undetectable viral load with ART. Although this supplemental protection is likely not necessary in light of U=U data supporting treatment-as-prevention, PrEP should be discussed as an option for prevention and offered when appropriate. It is important to note that the partner without HIV may choose to take PrEP for other reasons, including if they have additional sex partners, are unsure of a sex partner’s viral load or ability to maintain viral suppression, or feel more secure about and in control of their sexual health with the protection of PrEP.
Although the efficacy of TDF/FTC as PrEP during attempts to conceive has not been formally studied, it is an option for individuals who do not have HIV, and evidence suggests that it does not affect male fertility Were, et al. 2014 and is safe during the periconception period Mugo, et al. 2014. TAF/FTC has not been studied in the context of attempts to conceive.
People who inject drugs: The Bangkok Tenofovir Study is the only randomized controlled trial of PrEP in people who inject drugs Choopanya, et al. 2013. PrEP efficacy with TDF (alone) in this study was 49%, although restricting analysis to those with detectable drug in their blood increased efficacy to 74%.
PrEP for Adolescents
| NEW YORK STATE LAW |
|
Modeling studies have shown the potential for PrEP to be highly effective at reducing HIV incidence among adolescent MSM, directly through use by adolescents and indirectly by reducing HIV prevalence among their sex partners Hamilton, et al. 2019; Goodreau, et al. 2018.
TDF/FTC: In May 2018, the U.S. Food and Drug Administration (FDA) approved the use of TDF/FTC as PrEP in adolescents weighing ≥35 kg FDA 2018. The Centers for Disease Control and Prevention and the International Antiviral Society–USA previously extended the indication for TDF/FTC to include PrEP for adolescents at increased risk of acquiring HIV CDC(b) 2018; Marrazzo, et al. 2014.
To date, there is no evidence of increased TDF/FTC toxicity in adolescents taking this combination as part of an ART regimen. TDF/FTC as PrEP was safe and effective in adolescents, with no renal events or bone fractures noted Hosek, et al. 2017. Concerns regarding bone loss in younger age groups have been raised, with 2 studies reporting a decline in bone mineral density Havens, et al. 2017. Bone density changes associated with TDF use are reversible on discontinuation in adults and MSM 18 to 22 years old Hosek, et al. 2017. Data on bone density recovery after discontinuation of TDF/FTC as PrEP are not available for adolescents <18 years old. Studies are in progress to determine the safety of TDF/FTC for adolescents over long periods of time.
TAF/FTC: In October 2019, the FDA approved use of this regimen as PrEP in adults and adolescents weighing ≥35 kg FDA(a) 2019. There are no specific data on bone safety in adolescents taking TAF/FTC as PrEP; however, given the more favorable bone biomarkers of TAF compared with TDF, TAF may have an advantage in MSM and transgender female adolescents who have not achieved bone maturation, but this advantage is theoretical. Without clinical data, a clear recommendation cannot be made at this time.
CAB LA: In December 2021, CAB LA was approved by the FDA for use in adolescents ≥12 years old weighing ≥35 kg FDA(b) 2021.
Assessment and Counseling Before PrEP Initiation
| SELECTED GOOD PRACTICE REMINDERS |
Assessment and Counseling Before PrEP Initiation
|
|
Note:
|
Engagement in primary care: PrEP is an integral part of sexual health and well-being. Developing an HIV prevention plan that includes PrEP offers care providers the opportunity to engage individuals in primary care. Clinicians may use this opportunity to encourage age-appropriate health screenings, substance use screening and interventions, linkage to specialty services, and other health maintenance activities, such as immunizations (e.g., hepatitis A and B vaccines, human papillomavirus vaccine for patients ≤45 years old, and meningococcal vaccine when appropriate).
Patient education: Patient education is vital to shared decision-making and the success of PrEP as part of a comprehensive HIV prevention plan. Educate candidates about risks, benefits, and the choice of oral versus injectable PrEP. Discuss individual preferences, needs, and circumstances. Adherence may improve when patients participate in medication-related decisions Johnson, et al. 2012 and are informed about the strong efficacy of PrEP when taken as directed (see guideline sections Choosing and Prescribing a PrEP Regimen > Engagement in Care and Adherence). Education provided in the individual’s native or preferred language and tailored to the individual’s level of comprehension will help ensure understanding of:
- How PrEP works
- The benefits and risks of PrEP
- The need for adherence to the dosing schedule for PrEP to be protective
- The importance of regular monitoring and adherence to the visit schedule
- How safer sex or safer drug injection practices decrease the risk of pregnancy and the risk of acquiring drug-resistant HIV, other sexually transmitted infections (STIs), and hepatitis C virus (see Be in the KNOW > Sex and HIV and NYSDOH Syringe Access and Disposal).
Health literacy assessment: Use a health literacy assessment to evaluate the individual’s knowledge of the:
- Purpose of PrEP
- Importance of adherence to PrEP
- Importance of scheduled HIV testing and routine monitoring
- Potential adverse effects of PrEP
- Process for obtaining regular pharmacy refills for PrEP
- Methods of paying for PrEP or access to payment assistance for PrEP medications and related care services
See An Introduction to Health Literacy from the National Library of Medicine for more details on the various aspects of health literacy, including tools for assessing health literacy.
Sex and drug use histories: A detailed HIV risk assessment includes obtaining a patient’s sexual history and drug use history and having a frank, open, and nonjudgmental discussion of risk-related behaviors. As indicated, this discussion may also include the offer of further counseling and referrals, such as for substance use treatment (see NYSDOH Office of Addiction Services and Supports: Treatment).
Status of sex partner(s) with HIV: The ART and viral load status of a sex partner with HIV may inform the discussion of risk. Sexual transmission of HIV does not occur when an individual with HIV has a persistently undetectable HIV viral load; nonetheless, an individual without HIV in a serodifferent partnership who does not have HIV may still elect to use PrEP Prevention Access Campaign 2019; NYSDOH 2017; Rodger, et al. 2016. If the patient’s partner has detectable virus and genotypic information is unavailable, knowledge of the partner’s ART regimen may be helpful. The risk of acquisition is increased when an individual is exposed to HIV that is resistant to the components of their PrEP regimen Knox, et al. 2017; NYC Health 2016. The potential for drug resistance is an important consideration when choosing a PrEP regimen. If there is a risk of drug resistance in a sex partner with HIV, it is essential to advise the individual without HIV to use additional prevention measures, including condoms.
Reproductive counseling: Inquire about the patient’s reproductive plans and provide preconception counseling when indicated. Determine whether the patient or the patient’s partner is pregnant or breastfeeding, intends to conceive, or is currently using hormonal or other contraception in addition to condoms Bujan and Pasquier 2016; Lampe, et al. 2011; Vernazza, et al. 2011. Counsel HIV-serodifferent couples who are considering the use of PrEP during attempts to conceive about the utility, safety, and possible risks of the medication (see guideline section Choosing and Prescribing a PrEP Regimen > PrEP During Pregnancy).
Psychosocial assessment: Assessments of psychosocial needs, strengths, challenges, mental health, and substance use are integral to good general medical practice. In the case of someone prescribed PrEP, such assessments enable clinicians to identify modifiable barriers to adherence and provide services and referrals to support adherence and retention in care.
| PrEP PAYMENT ASSISTANCE |
|
PrEP in Comprehensive HIV Prevention Planning
A comprehensive HIV prevention plan includes counseling and education about PrEP options, adherence to PrEP, ongoing monitoring with laboratory tests, education about risk reduction, and discussion of additional HIV prevention options, including the use of condoms and safe drug injection practices Blashill, et al. 2015; Daughtridge, et al. 2015; Liu, et al. 2014; Marcus, et al. 2014.
Risk reduction: At every visit, clinicians should encourage risk reduction through condom use, safer sex practices, and if applicable, safer injection techniques Bramson, et al. 2015; Abdul-Quader, et al. 2013; Jarlais 2013. Discussions about risk reduction should be tailored to patients’ specific needs. However, condom use is not a prerequisite for PrEP use.
Decreased condom use has been observed in some individuals taking PrEP and has been associated with a concomitant rise in other viral and bacterial STIs for which PrEP offers no protection; however, this is not a valid reason to withhold PrEP (see guideline section Ongoing Laboratory Testing > Routine Laboratory Testing > STI screening) Traeger, et al. 2018; Werner, et al. 2018; Hojilla, et al. 2016; Kuhns, et al. 2016; Golub 2014; Liu, et al. 2013.
Risk compensation: Increased engagement in high-risk sexual behaviors, such as condomless sex or multiple sex partners, can lead to an increase in STI incidence and is sometimes cited by care providers as a reason not to offer PrEP. A meta-analysis of 20 studies found that rates of sexual risk behaviors and STIs in MSM taking PrEP remained stable or decreased in the majority of the studies Werner, et al. 2018. In a separate meta-analysis limited to open-label studies in which participants knew they were receiving active drug, a majority of the studies reported an increase in condomless sex but no significant increase in the proportion of MSM participating in condomless sex, indicating that participants were not using condoms consistently before they started PrEP Traeger, et al. 2018.
High baseline STI rates in PrEP clinical trial participants demonstrate that risk behavior often precedes engagement in PrEP care. Initiating PrEP care for individuals at risk for HIV provides an opportunity for routine STI screening and treatment. One modeling study demonstrated that increased PrEP engagement along with routine STI screening and treatment would lower STI rates through detection and treatment of asymptomatic STIs that might otherwise remain undiagnosed. Modeling indicated that STI rates decline more rapidly as higher numbers of at-risk individuals initiate PrEP and related STI screening services, with an even greater reduction in STIs the more frequently STI testing occurs, even in the event of a 40% to 80% decrease in condom use Jenness, et al. 2017.
PrEP after PEP: Patients who remain at increased risk of HIV exposure after completing a course of nPEP and who are negative for HIV at the 4-week test should be offered PrEP to begin immediately after the last dose of nPEP.
| KEY POINTS |
|
Contraindications to PrEP Use
HIV infection: The 2-drug PrEP regimens of TDF/FTC and TAF/FTC and the single-drug regimen of CAB LA are not adequate for treating established HIV infection; therefore, PrEP should not be initiated unless an individual is tested for HIV within 1 week before the proposed initiation. If HIV infection is confirmed, PrEP should immediately be converted to a fully suppressive HIV treatment regimen. For more information, see the NYSDOH AI guidelines Rapid ART Initiation and Selecting an Initial ART Regimen.
Renal dysfunction: TDF can cause renal toxicity and is contraindicated for patients with a CrCl <60 mL/min at the time of PrEP initiation FDA 2016. There are no data for adjusting TDF dosing in those with an estimated CrCl <50 mL/min.
TAF/FTC can be initiated in individuals with a creatinine clearance ≥30 mL/min and can be used in cisgender MSM and transgender women who have a CrCl <60 mL/min on initiation or a CrCl that drops to <50 mL/min while taking TDF/FTC.
Increased monitoring for adverse effects is appropriate for individuals with a CrCl <30 mL/min receiving CAB LA. Serum creatinine levels can vary and be affected by factors other than renal disease; therefore, before a decision is made to forgo oral PrEP, decreased CrCl should be verified through repeat testing, and other causes of spurious creatinine elevation (e.g., use of creatine-containing protein supplements) should be ruled out. Potentially reversible causes, such as the use of nonsteroidal anti-inflammatory medications, may also be addressed.
CAB LA is an appropriate PrEP option for individuals who cannot take a tenofovir-containing regimen because of renal dysfunction. To decrease drug exposure, on-demand dosing of TDF/FTC may also be considered when appropriate for cisgender MSM with borderline renal function or other kidney disease with a preserved calculated CrCl (see guideline section Choosing a Preferred PrEP Regimen > Preferred Oral Regimen for Daily or On-Demand Dosing: TDF/FTC).
When medication cannot be used for PrEP, education regarding other prevention options, such as condom use and safer sex practices, is essential.
PrEP Failure
HIV acquisition despite adherence to PrEP is rare. PrEP failure is directly related to suboptimal adherence in all but a small number of cases. In most cases of HIV acquisition despite adherence to PrEP, there was unrecognized HIV infection at the time of PrEP initiation.
There are case reports of individuals who acquired HIV despite adherence to TDF/FTC as PrEP, measured by tenofovir concentration in hair or dried blood spot samples. These case reports have noted that some individuals were exposed to HIV that was resistant to tenofovir and emtricitabine Knox, et al. 2017; NYC Health 2016. One individual acquired HIV with mutations that should still have conferred sensitivity to TDF/FTC Cohen, et al. 2019. Another acquired wild-type virus while using PrEP Hoornenborg(a), et al. 2017. It is theorized that in the case of wild-type virus acquisition despite good adherence, exposure to HIV was potentially very high given the number of condomless exposures over the 6-month period and the presence of rectal STIs.
PrEP failures with CAB LA were rare and mainly occurred before the initiation of study drug, during oral lead-in, in individuals who missed a scheduled injection, or during the tail phase after treatment was discontinued. In the HPTN 083 study, 6 individuals acquired HIV despite on-time CAB LA injections Landovitz, et al. 2022; Landovitz, et al. 2021. Analysis is under way to better understand these failures. In the HPTN 084 study, there were no incident HIV infections with on-time CAB LA injections in cisgender women Marzinke(b), et al. 2021.
Although PrEP failure with CAB LA is rare, integrase resistance mutations were found in 5 of 9 participants with a resistance test result in the HPTN 083 study Marzinke(a), et al. 2021. There were 4 PrEP failures with CAB LA in the HPTN 084 study, with one infection occurring in an individual who received on-time injections. There was no finding of integrase resistance among these participants Marzinke(b), et al. 2021. There is concern with long-acting injectable medications about the slow decay in drug levels over time once the medication is stopped and that individuals who acquire HIV during the CAB LA tail phase will develop integrase resistance. It is reassuring that in the HPTN 083 study, none of the individuals who acquired HIV during the tail phase had integrase resistance mutations.
Choosing and Prescribing a PrEP Regimen
| RECOMMENDATION |
Choice of Regimen
TDF/FTC
TAF/FTC
Patients With HBV Infection
CAB LA
|
Resource: Appendix: Care Provider Checklists for PrEP Initiation, Regimen Choice, and Follow-Up Abbreviations: CAB LA, long-acting injectable cabotegravir (brand name Apretude); CrCl, creatinine clearance; HBV, hepatitis B virus; IM, intramuscular; INSTI, integrase strand transfer inhibitor; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (brand name Truvada). |
Comparison of Available Regimens
All current PrEP options are highly effective when taken as directed. Shared decision-making allows patient preferences and clinical considerations to guide the choice of the best PrEP option for each candidate (see Tables 1 and 2, below).
| Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; CAB, cabotegravir; CrCl, creatinine clearance; FDA, U.S. Food and Drug Administration; FTC, emtricitabine; HBV, hepatitis B virus; IM, intramuscular; LDL, low-density lipoprotein; MSM, men who have sex with men; NYCRR, New York Codes, Rules and Regulations; PEP, post-exposure prophylaxis; PK, pharmacokinetic; PrEP, pre-exposure prophylaxis; TAF, tenofovir alafenamide; TasP, treatment-as-prevention; TDF, tenofovir disoproxil fumarate.
Notes:
|
|||
| Table 1: Comparison of Key Clinical and Logistical Factors in Choosing a PrEP Regimen (details provided in discussion that follows; also see Appendix: Care Provider Checklists for PrEP Initiation, Regimen Choice, and Follow-Up) | |||
| TDF/FTC (tenofovir disoproxil fumarate/emtricitabine; Truvada) |
TAF/FTC (tenofovir alafenamide/emtricitabine; Descovy) |
CAB LA (long-acting injectable cabotegravir; Apretude) |
Comments |
| Efficacy | |||
| All exposures, including sexual and injection drug use |
|
|
A 2017 amendment to the NYCRR grants minors capacity to consent to PrEP and PEP without parental/guardian involvement |
| Time to Protection [b] | |||
|
No data | No data | — |
| Renal Safety | |||
|
|
Increased monitoring for adverse effects is recommended with CrCl <30 mL/min |
|
| Bone Safety | |||
| Potential decrease in bone mineral density; meta-analysis shows good safety [c] |
|
Preferred option for prevention of sexual exposures in all individuals with osteopenia or osteoporosis | Inform patients with preexisting risk factors or documented osteopenia, osteomalacia, or osteoporosis of the risk of bone loss with TDF/FTC; weigh the risks and benefits |
| Weight and LDL Cholesterol | |||
|
|
|
— |
| Dosing | |||
|
Daily dosing only |
|
— |
| Same-Day Initiation | |||
| Generic TDF/FTC is a preferred insurance option and is usually available for same-day initiation | May require prior authorization |
|
— |
| Common Adverse Effects | |||
| Diarrhea (6%), nausea (5%) [d] | Diarrhea (5%), nausea (4%) [e] | Injection site reactions (32% to 81%) [f], which are mostly mild and greatest initially | — |
| Use During or When Planning Pregnancy | |||
|
Do not use for vaginal exposure; no data in pregnancy |
|
|
| Use With Oral Contraceptives | |||
| No interaction expected based on PK data | Not for use as PrEP for vaginal sexual exposure | No interaction expected based on PK data | — |
| Use With Gender-Affirming Hormones | |||
|
No data; no interaction expected based on PK profiles and lack of significant interactions with oral contraceptives | No data; no interaction expected based on PK profiles and lack of significant interactions with oral contraceptives | — |
| Patients With Active Chronic HBV [g,h] | |||
|
|
Not active against HBV infection | Monitor closely for rebound HBV viremia if TDF/FTC or TAF/FTC is discontinued in a patient with chronic HBV infection |
| Drug-Drug Interactions | |||
| See NYSDOH AI Resource: ART Drug-Drug Interactions > TDF and TAF Interactions | See NYSDOH AI Resource: ART Drug-Drug Interactions > TDF and TAF Interactions | See NYSDOH AI Resource: ART Drug-Drug Interactions > CAB Interactions | — |
| Generic Formulation Availability | |||
| Generic TDF/FTC is available | Brand only | Brand only | TAF/FTC and CAB may require prior insurance authorization |
| Abbreviations: CAB LA, long-acting injectable cabotegravir (brand name Apretude); HBV, hepatitis B virus; HSV, herpes simplex virus; IM, intramuscular; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (brand name Truvada).
Note:
|
||
| Table 2: Comparison of Benefits, Limitations, and Risks of Available PrEP Regimens | ||
| All PrEP Regimens | Oral PrEP With TDF/FTC or TAF/FTC | Injectable PrEP With CAB LA |
| Benefits | ||
|
|
|
| Limitations | ||
|
|
|
| Risks | ||
|
|
|
Time to Protection
The time to protection against HIV infection after PrEP initiation is not definitively established. No studies have directly assessed time to protection, and the site of PrEP action in blood, rectal, and genital tissues has not been settled. Most of what is known is from animal studies, human tissue studies, and pharmacokinetic modeling. There are no studies with clinical endpoints. In animal models and observational studies, post-exposure prophylaxis has been effective if initiated 36 to 72 hours after exposure, which raises questions about the specific drug concentrations in tissue required to protect against HIV infection.
TDF/FTC: Early pharmacokinetic modeling data demonstrated that 7 days of daily TDF/FTC for PrEP are required to achieve maximal protective concentrations in rectal tissue, and 20 days of daily dosing are required to achieve maximal protective concentrations in cervicovaginal tissue Louissaint, et al. 2013; Anderson, et al. 2012; Patterson, et al. 2011. No data are available on protective concentrations of TDF/FTC in penile tissue. Based on these data, the 2017 Centers for Disease Control and Prevention and NYSDOH AI PrEP guidelines recommended 7 days of TDF/FTC as PrEP for rectal exposure and 20 days of PrEP for vaginal, penile, and injection exposures to achieve protection.
Other pharmacokinetic modeling data suggest that TDF/FTC is likely protective within 1 week of dosing in rectal and genital compartments and peripheral blood mononuclear cells (PBMCs) Hendrix, et al. 2016; Seifert, et al. 2016. Emtricitabine triphosphate (active metabolite of emtricitabine) reaches steady-state and therapeutic concentrations very quickly in vaginal tissue, and tenofovir diphosphate (active metabolite of tenofovir) reaches concentrations more slowly, potentially affording vaginal protection quite early from emtricitabine triphosphate while tenofovir diphosphate concentrations accumulate Cottrell, et al. 2016; Seifert, et al. 2016.
Some guidelines have suggested shorter intervals to PrEP protection with TDF/FTC. Both the 2017 World Health Organization (WHO) and the 2018 British HIV Association (BHIVA) guidelines recommend a 7-day PrEP lead-in for protection against HIV via vaginal and injection exposures BHIVA 2019; WHO 2017. The WHO guidelines recommend a time to protection of 7 days for all sites of exposure, but the BHIVA guidelines advise that protection against rectal exposure is achieved 24 hours after an initial double (loading) dose, based on the efficacy shown in the IPERGAY study of on-demand TDF/FTC and pharmacokinetics indicating achievement of protective concentrations of TDF/FTC sooner with a loading dose. Although concrete guidance on efficacy would be best, and accumulating evidence points to an earlier time to protection for vaginal exposure, a definitive answer to this question is not yet available, and experts disagree in their interpretation of the evidence AVAC 2017.
TAF/FTC: Time to protection for TAF/FTC is also unclear. TAF had a faster time to 90% effective concentrations (EC90) than TDF (4 hours vs. 3 days) and significantly higher steady-state concentrations in PBMCs than TDF/FTC as PrEP Ogbuagu, et al. 2021, which may confer a clinical benefit in terms of time to protection and adherence forgiveness, but this is preliminary evidence and further evaluation is needed.
CAB LA: At this time, there are no data available on time to protection for CAB LA, and specific guidance cannot be provided.
| KEY POINTS |
|
Preferred Oral Regimen for Daily or On-Demand Dosing: TDF/FTC
When TDF/FTC is preferred: TDF/FTC is the preferred oral regimen for PrEP because of its proven efficacy and safety in clinical trials, its suitability for use as PrEP in most populations (including individuals who inject drugs), and its cost, which is lower than that of TAF/FTC. However, for cisgender MSM and transgender women with preexisting renal disease or osteoporosis, TAF/FTC is the preferred oral regimen; see below.
Daily dosing: The FDA-approved dosing of TDF/FTC as PrEP is 1 tablet daily by mouth with or without food FDA 2016.
For cisgender MSM, the data on daily dosing of TDF/FTC are more robust than for on-demand dosing, with longer follow-up. Daily dosing remains the preferred dosing strategy.
On-demand dosing: For TDF/FTC as PrEP, an on-demand dosing strategy (also called intermittent, event-driven, or coitally timed PrEP) is an option for MSM when lifestyle, sexual practices, or stated preferences make it a more acceptable choice.
On-demand dosing of TDF/FTC is not recommended for protection against vaginal sexual exposure, blood exposure through injection drug use, or in individuals with HBV infection. It should be used with caution in transgender women taking gender-affirming hormones.
When used on-demand, TDF/FTC is taken as a “2-1-1” regimen:
- 2 to 24 hours before sex: Take 2 TDF/FTC tablets (closer to 24 hours is preferred), followed by
- 24 hours after sex: Take 1 TDF/FTC tablet, then
- 48 hours after sex: Take 1 TDF/FTC tablet
- If sex occurs again: Take 1 TDF/FTC tablet daily until 48 hours after the last sex act, effectively becoming daily PrEP for as long as sex continues.
Considerations for on-demand PrEP: Successful on-demand PrEP requires planning ahead for sex by at least 2 hours. Patients have to review their usual practices in planning for sex to assess the feasibility of this approach, and clinicians have to ensure that patients understand the complex dosing schedule.
The logistical challenge of determining the amount of medication to prescribe for patients taking PrEP on demand should not preclude using this strategy when it is appropriate. Switching back and forth between daily and on-demand PrEP may be an appealing, evidence-based option for some individuals Molina, et al. 2022; Hoornenborg(b), et al. 2017.
Evidence for the efficacy of on-demand dosing includes the results of the IPERGAY and Prevenir studies, which found that “2-1-1” dosing effectively prevented HIV acquisition in MSM Molina, et al. 2015. Concerns have been raised as to whether the efficacy observed in the IPERGAY study resulted from the frequency of dosing when PrEP was used on-demand in this study. The average number of PrEP doses taken per month by participants in the IPERGAY study was 15 (approximately 4 doses per week) Molina, et al. 2015, which was the minimum level of adherence necessary for protection in the iPrEx study Anderson, et al. 2012. The iPrEx-OLE study, an open-label extension of the iPrEx study, confirmed that 4 or more doses of TDF/FTC as PrEP per week were protective Grant, et al. 2014. However, in a substudy of IPERGAY, PrEP dosed on demand remained effective even in participants with less frequent sexual activity Antoni, et al. 2020. The HPTN 067 ADAPT study investigated daily versus time-driven (twice per week with an additional dose after sex) versus event-driven dosing and found that adherence with event-driven dosing was lower than daily dosing Velloza, et al. 2019.
TDF/FTC as PrEP for transgender women: Studies have shown that estrogen use may lower tenofovir levels in the plasma of transgender women compared with cisgender men, but more recent studies call these findings into question. Levels of adherence needed for transgender women using estrogen and taking TDF/FTC for PrEP may be higher than for cisgender men, but the drug concentrations achieved with daily dosing of TDF/FTC as PrEP will confer protection (see guideline section PrEP for Individuals at Risk of Acquiring HIV > PrEP Candidates > Transgender women).
The significance of this potential difference in tenofovir concentrations with regard to on-demand dosing for transgender women is less clear; therefore, on-demand dosing cannot be uniformly recommended for this population. However, if neither daily oral dosing nor injections are viable options for a transgender woman at high risk of acquiring HIV, it is reasonable to discuss the risks and benefits of on-demand dosing, including the lack of data, and engage the patient in informed, shared decision-making regarding this dosing strategy.
Not recommended: The effectiveness of on-demand TDF/FTC as PrEP for vaginal exposure has not been established. In the HPTN 067 ADAPT study, levels of orally administered tenofovir were much lower in the vagina than in the anal compartment Anderson, et al. 2016. Other data suggest that vaginal sex requires nearly 100% adherence to PrEP to achieve protective levels Cottrell, et al. 2016. Without more data, the differential pharmacokinetics for vaginal exposure with TDF/FTC as PrEP precludes a recommendation for on-demand dosing for cisgender women or transgender men engaging in receptive vaginal sex. Data are also lacking for men who have sex with women.
On-demand dosing cannot be recommended for protection against blood exposure. Based on data from the Bangkok Tenofovir Study, which studied TDF alone in people who inject drugs, it appears that the rate of PrEP adherence required for protection must be higher for people who inject drugs than for other populations Choopanya, et al. 2013.
Common adverse effects: In clinical trials of TDF/FTC as PrEP, the most common adverse effects were nausea, headache, abdominal pain, and dizziness, which were mild and short-lived McCormack, et al. 2016; Thigpen, et al. 2012; Grant, et al. 2010. Most adverse effects peaked at 1 month and generally resolved within 3 months Glidden, et al. 2016. In a study comparing TAF/FTC and TDF/FTC as PrEP, both regimens were well tolerated, with diarrhea the only adverse effect in >10% of individuals in either arm Mayer, et al. 2020.
Renal impairment and loss of bone density: Both have been observed in individuals taking TDF/FTC as treatment for HIV, predominantly in studies of TDF/FTC combined with a third drug containing the pharmacokinetic booster ritonavir or cobicistat. Studies comparing unboosted TDF/FTC and TAF/FTC for HIV treatment found no difference in adverse events between the 2 arms Hill, et al. 2018. A meta-analysis of 13 trials of TDF/FTC used as PrEP found no difference in serious adverse events between TDF/FTC and placebo Pilkington, et al. 2018. The meta-analysis did find a borderline statistically significant difference in creatinine elevation of all grades when comparing TDF/FTC with placebo and adding in grade 1 and 2 elevations.
The DISCOVER study, which compared TDF/FTC with TAF/FTC for PrEP, did find a small but statistically significant difference between biomarkers of renal and bone dysfunction favoring TAF/FTC, although no difference was found in clinical adverse outcomes Mayer, et al. 2020. Although renal dysfunction is uncommon in individuals taking TDF/FTC as PrEP, and especially those who are younger Gandhi, et al. 2016, regular laboratory monitoring is necessary during use of TDF/FTC or TAF/FTC (see Table 4: Recommended Routine Laboratory Testing for Patients Taking PrEP). If an increase in serum creatinine or a decrease in calculated CrCl is observed, evaluate potential causes other than TDF or TAF use. Discontinuation or interruption of TDF/FTC as PrEP is appropriate if other causes are ruled out or if CrCl drops to <50 mL/min (confirmed on 2 readings) for any reason. TAF/FTC is indicated for cisgender MSM and transgender women with CrCl ≥30 mL/min. When appropriate, on-demand dosing of TDF/FTC can also be considered to decrease drug exposure for MSM with borderline renal function.
Bone density losses with TDF/FTC as PrEP are minimal and have not been associated with bone fractures Havens, et al. 2020; Spinelli, et al. 2019. No additional monitoring of bone mineral density is recommended.
For cisgender MSM and transgender women at high risk of fracture due to osteoporosis or at increased risk of developing osteoporosis, TAF/FTC is the preferred oral regimen and should be considered.
| KEY POINT |
|
Alternative Oral Regimen for Daily Dosing: TAF/FTC
TAF/FTC is an alternative PrEP regimen for cisgender MSM and transgender women.
When TAF/FTC is preferred: It is the preferred oral regimen for cisgender MSM and transgender women with preexisting renal disease or osteoporosis, and it may be preferable in MSM and transgender women with multiple risk factors for renal disease or osteoporosis.
Daily dosing: The FDA-approved dosing of TAF/FTC as PrEP is 1 tablet daily by mouth with or without food FDA(a) 2019.
On-demand dosing: On-demand dosing has not been studied for TAF/FTC; this regimen should not be dosed on-demand in any population.
TAF/FTC was noninferior to TDF/FTC in cisgender MSM and in the small group of transgender women who participated in the DISCOVER trial, a large, double-blinded, active-control study Mayer, et al. 2020. In that trial, the TAF component was associated with improved biomarkers for renal and bone safety; however, the clinical significance of these changes is unclear, and there was no difference in clinical outcomes regarding adverse events. TAF use was associated with small increases in weight gain and low-density lipoprotein (LDL) cholesterol levels compared with TDF, but the clinical significance of these differences is unclear Mayer, et al. 2020; Orkin, et al. 2018.
TAF may have an advantage in MSM and transgender female adolescents who have not achieved bone maturation, given favorable bone biomarkers compared with TDF, but this advantage is theoretical, and without clinical data, a clear recommendation cannot be made at this time Havens, et al. 2020; Mayer, et al. 2020; Spinelli, et al. 2019; Hosek, et al. 2017.
TAF/FTC has not yet been studied for vaginal exposures in human trials. Study results to date suggest that tenofovir concentrations in vaginal tissue after administration of TAF are lower than for TDF Cottrell, et al. 2017, although TAF does reach high intracellular concentrations in PBMCs, and oral TAF/FTC was effective in preventing vaginal simian HIV infections in macaques Massud, et al. 2019. Further study of TAF/FTC for protection in vaginal and injection HIV exposures is needed. TAF/FTC has also not been studied in insertive partners in the context of vaginal sex.
Common adverse effects: In a study comparing TAF/FTC and TDF/FTC as PrEP, both regimens were well tolerated, with diarrhea the only adverse effect in >10% of individuals in either arm Mayer, et al. 2020.
| KEY POINTS |
|
Manage adverse effects: Two weeks after oral PrEP initiation, a care team member should follow up either in person or by telephone to assess and address adverse effects and offer advice for management until they abate. Gastrointestinal adverse effects can be alleviated by taking PrEP medications with food or antidiarrheal agents, anti-gas medications, and antiemetics. In the iPrEx Grant, et al. 2014; Grant, et al. 2010 and Partners Mujugira, et al. 2016 trials of TDF/FTC as PrEP, rash was not reported as a common adverse effect. Patients who develop a rash while taking TDF/FTC or TAF/FTC as PrEP should be assessed for syphilis and acute HIV Apoola, et al. 2002.
Preferred Injectable Regimen: CAB LA Every 2 Months
In December 2021, the FDA approved CAB LA for use as PrEP for sexual exposures in adults and adolescents weighing ≥35 kg FDA(b) 2021.
HPTN 083, a study of a diverse multinational population of 4,566 MSM and transgender women, found CAB LA to be statistically superior to TDF/FTC in MSM and transgender women Landovitz, et al. 2021. HPTN 084, a study of 3,224 cisgender women in 7 sub-Saharan African countries, also found CAB LA to be statistically superior to TDF/FTC Delany-Moretlwe, et al. 2022. Both studies found CAB LA to be safe and well tolerated, with the main adverse effects being injection site reactions, which were generally mild and decreased in incidence over time.
When CAB LA is preferred: CAB LA is a preferred agent for protection against sexual exposure to HIV in adults and adolescents weighing ≥35 kg.
CAB LA is a preferred agent for individuals who are open to injectable PrEP, because of its statistically superior efficacy in preventing HIV compared with TDF/FTC and its protection against HIV through all types of sexual exposure Delany-Moretlwe, et al. 2022; Landovitz, et al. 2021. CAB LA is a good option when oral medication poses a challenge to PrEP use.
Oral CAB lead-in: In clinical trials of CAB LA, a 5-week oral CAB lead-in was administered to rule out adverse effects before patients received a long-acting injection Delany-Moretlwe, et al. 2022; Landovitz, et al. 2021. There are no data on the safety or efficacy of CAB LA when used for PrEP without an oral lead-in.
However, in clinical trials of injectable long-acting CAB plus rilpivirine (CAB/RPV LA) for HIV-1 treatment, omission of the oral lead-in was safe and did not interfere with achievement of adequate plasma CAB levels when injections were initiated Orkin, et al. 2021. There were no CAB safety concerns identified during the oral lead-in phase in trials of CAB/RPV LA as HIV treatment Rizzardini, et al. 2020 or in trials of CAB as PrEP. As a result, the oral lead-in phase for CAB LA as PrEP is now optional.
Some care providers or patients concerned about ensuring tolerability before initiating a long-acting treatment may prefer an oral CAB lead-in. An important consideration in such cases is the potential risk of HIV acquisition for individuals who may struggle with adherence to daily oral medication. Of note, 3 of the 12 incident HIV infections in individuals using CAB as PrEP in the HPTN 083 study were acquired during the oral lead-in phase Marzinke(a), et al. 2021. Ongoing daily oral CAB is not FDA-approved or recommended as PrEP.
TDF/FTC (or TAF/FTC if appropriate) can be used as an alternative oral lead-in therapy for same-day initiation when oral CAB or CAB LA is not immediately available because of insurance or logistics issues. Some care providers may choose to briefly overlap oral TDF/FTC or TAF/FTC with CAB to maintain protection against HIV while CAB levels are attaining steady-state.
Oral CAB as bridge therapy: Oral CAB can also be used as bridge therapy when it is anticipated that an injection will be missed. Oral CAB is currently only available from a central pharmacy in collaboration with ViiV Healthcare. TDF/FTC (or TAF/FTC if appropriate) can also be used as bridge therapy when logistics impede timely access to oral CAB.
CAB LA injections: CAB LA as PrEP is administered as a 600 mg IM injection every 2 months after the first 2 injections are given 4 weeks apart. See Box 2, below, for details on the preparation and administration of CAB LA as PrEP.
The median time from the last injection to when CAB LA concentrations decreased below the lower limit of quantification was 43.7 weeks for male participants and 67.3 weeks for female participants in the HPTN 077 trial Landovitz, et al. 2020. Because of the long decay time for CAB LA to be eliminated, if a patient chooses to discontinue CAB LA and there is an ongoing risk for HIV exposure, they should be transitioned to an oral PrEP regimen 2 months after the last injection. If risk is ongoing, oral PrEP should be continued for at least 1 year to prevent the acquisition of INSTI-resistant HIV. However, it is reassuring that none of the patients in the HPTN 083 study who acquired HIV during the tail phase had INSTI resistance mutations Landovitz, et al. 2021.
| Box 2: Preparation and Administration of CAB LA as PrEP (see package insert) |
CAB LA as PrEP is given as a 3 mL (600 mg) deep IM gluteal injection. After the first injection, a second injection is administered 4 weeks later, after which injections are administered bimonthly (within 1 week before or after the next planned dose).
|
|
Abbreviations: CAB LA, long-acting injectable cabotegravir; IM, intramuscular; PrEP, pre-exposure prophylaxis. |
Adverse effects: In the clinical trials noted above, CAB LA as PrEP was well tolerated. Adverse reactions observed in at least 1% of participants included injection site reactions, diarrhea, headache, pyrexia, fatigue, sleep disorders, nausea, dizziness, flatulence, abdominal pain, vomiting, myalgias, rash, decreased appetite, somnolence, back pain, and upper respiratory tract infection; however, almost all were grade 1 level effects. The most common adverse effects were injection site reactions in 81% of HPTN 083 study participants and 32% of HPTN 084 study participants. Reactions were mostly mild or moderate in intensity and decreased in frequency and intensity over time. Median onset was 1 day after injection in the HPTN 083 trial and lasted a median of 3 days. Discontinuations due to injection site reactions were rare and occurred at a rate of 2.4% in the HPTN 083 trial and 0.0% in the HPTN 084 trial. Other studies have reported a strong preference for long-acting injectable medications despite injection site reactions Tolley, et al. 2020; Murray, et al. 2018.
Metabolic effects: Mild weight gain was observed in MSM and transgender women receiving CAB LA as PrEP compared with TDF/FTC, but there was no significant difference in weight between the 2 study arms in cisgender women, and there was no significant effect on lipid levels Delany-Moretlwe, et al. 2022; Landovitz, et al. 2021.
CAB LA implementation: The logistics of implementing an injectable option in PrEP programs may be challenging and requires institutional, clinician, and patient preparation. Medication storage, scheduling and reminders, tracking systems, and staffing levels must be addressed with implementation plans appropriate to each setting (see Box 3, below).
| Box 3: Implementation Strategies for Long-Acting Injectable Cabotegravir as PrEP |
Institutional and clinician preparations:
Patient preparations:
|
|
Abbreviation: PrEP, pre-exposure prophylaxis. |
Engagement in Care
Engagement in care is a challenge for PrEP programs. Flexibility in visit frequency and provision of alternative modalities, such as telehealth, can enhance care access and engagement. The availability of both oral and injectable PrEP options increases choices for individuals and may also enhance persistence in care.
Challenges: Multiple studies have shown that persistence on PrEP remains a challenge Wagner, et al. 2023; Blumenthal, et al. 2021; Chan, et al. 2016. One study that examined PrEP programs in 3 midsized cities found the rate of persistence in PrEP care at 6 months to be 60%, owing to individual and structural barriers Chan, et al. 2016. “Overmedicalization” of PrEP care may pose a barrier to persistence by requiring healthy individuals to engage frequently with health care through at least quarterly clinic visits and laboratory tests. Although quarterly assessments remain the standard for oral PrEP monitoring, flexibility regarding in-person visits is encouraged when needed or appropriate. Quarterly laboratory testing is recommended even with a decision to adjust visit frequency, but flexibility for individual patients regarding this time frame is appropriate because quarterly screening is a best practice rather than an evidence-based recommendation. Barriers to engagement in care should be explored with all patients, and individualized solutions should be explored with those struggling to remain in care who wish to continue PrEP use. Novel models, such as annual visits with quarterly at-home HIV testing Siegler, et al. 2019, are promising, and telemedicine visits should be encouraged when appropriate to remove structural barriers for patients having difficulty with PrEP persistence.
Injectable PrEP may increase PrEP uptake and persistence among individuals for whom regular oral medication is a barrier to care, those with preexisting renal disease, or those who prefer the convenience of an injection once every 2 months to daily oral PrEP. However, CAB LA as PrEP requires visits every 2 months for both monitoring and injections. This could be a barrier for patients who prefer visits only every 6 to 12 months (with quarterly laboratory tests in the interim), as indicated for current oral PrEP options, or who have life or work challenges that make visits every 2 months logistically challenging. Institutions will need sufficient appointment availability and flexibility in days and hours of care to increase access to care and persistence with CAB LA. Tracking systems and reminder calls can help increase adherence to injection visits. Alternative care models, including home visits for injections, will help increase access to this treatment.
Episodic PrEP: PrEP use may be noncontinuous, as individuals may cycle off and on PrEP based on fluctuations in their risk of acquiring HIV Reed, et al. 2021. Therefore, discontinuation of PrEP may not indicate program failure. However, for individuals who want to remain on PrEP, it is essential to remove barriers and individualize care.
Gender-affirming care: Transgender women have high rates of PrEP discontinuation Scott, et al. 2019. Providing gender-affirming and transition-related care to transgender individuals may increase PrEP uptake and persistence Sevelius, et al. 2016. Transgender women may avoid PrEP or miss doses because they are concerned that drugs active against HIV lower estrogen levels. Multiple studies have shown that TDF/FTC does not lower estrogen plasma concentrations Blumenthal, et al. 2022; Grant, et al. 2021; Hiransuthikul, et al. 2019. Addressing this directly with transgender women and providing reassurance regarding estrogen levels may improve their willingness to take and adhere to PrEP. Currently, there are no data directly evaluating the effect of TAF/FTC or CAB LA on estrogen plasma concentration; however, no interaction is expected based on the pharmacokinetic profiles of TAF/FTC and CAB LA and their lack of significant interactions with oral contraceptives.
Other services: Clinicians should partner with care providers within or outside of their organization to provide other services, including mental health and substance use treatment, case management, navigation and linkage services, housing assistance, and income/benefits assessments. Refer patients to support groups if indicated. However, PrEP should not be withheld from individuals who are not interested in primary care or who may choose to obtain PrEP from a location other than where they receive primary care.
| KEY POINTS |
|
Adherence
Challenges: Daily adherence to oral PrEP is challenging for some patients for a wide variety of complex and intersecting reasons, including pill counts and sizes, adverse effects related to PrEP medications, privacy concerns, PrEP-related stigma, neurocognitive disorders, mental health conditions, active substance use, psychological trauma, personal belief systems, travel requirements, occupation, and health literacy. Although the availability of a once-daily single-tablet oral option for PrEP is helpful to adherence, studies and real-world experience demonstrate ongoing challenges with adherence to daily oral PrEP for some individuals. The use of long-acting agents for PrEP can potentially address the adherence challenges that some individuals face, and studies have shown a high interest in long-acting agents for PrEP in the general population and in specific subpopulations Philbin, et al. 2021; Koren, et al. 2020; Rael, et al. 2020; Meyers, et al. 2014. CAB LA is the only long-acting injectable PrEP option currently approved by the FDA; however, other injectable and long-acting agents are under study.
The degree of adherence to TDF/FTC as PrEP required to prevent HIV varies by exposure site. Based on modeling studies of rectal exposure, 4 doses per week of TDF/FTC appears sufficient to protect against HIV Grant, et al. 2014; however, modeling studies also suggest that vaginal and injection HIV exposures require closer to 7 doses of TDF/FTC per week for efficacy Cottrell, et al. 2016; Choopanya, et al. 2013; Patterson, et al. 2011. There are no data on adherence needed for penile insertive exposure. Currently, no data are available regarding adherence levels required for TAF/FTC or CAB LA effectiveness.
Strategies for adherence support: In studies of TDF/FTC as PrEP, efficacy was highly dependent on adherence Sidebottom, et al. 2018, and on-time injections of CAB LA are essential to avoiding viral breakthrough Landovitz, et al. 2021. Some reasons for decreased adherence are adverse effects, fear of long-term toxicity, perceived low risk of infection, insurance issues, depression, substance use, and difficulty with daily dosing. For patients who struggle with adherence to PrEP, strategies such as more frequent visits or contact with medical and nonmedical care providers may be helpful. Adherence decreased when visits went from monthly to quarterly in adolescent patients Hosek, et al. 2017. Assessing and addressing individual reasons for suboptimal adherence to PrEP is important Goodreau, et al. 2018; Jenness, et al. 2016.
Some care providers use peer supporters to reinforce adherence to medication and appointments. If patients are consistently unable to adhere to an oral PrEP regimen despite interventions to improve adherence or if they decline to take oral PrEP daily, it may be appropriate to explore alternative dosing schedules, such as on-demand PrEP (for cisgender MSM only, with TDF/FTC), injectable PrEP, or seasonal or vacation use of PrEP. It may also be appropriate to discuss discontinuing PrEP and using other risk-reduction strategies that better meet the needs of the individual.
| KEY POINTS |
|
PrEP During Pregnancy
HIV acquisition risk is higher in pregnancy and is highest in the late pregnancy and early postpartum periods Thomson, et al. 2018, and PrEP should be recommended if the risk of HIV exposure continues. Risk of perinatal transmission is also significantly higher during pregnancy and breastfeeding in cases of acute seroconversion Drake, et al. 2014; Singh, et al. 2012. Encourage pregnant individuals to inform their obstetric and pediatric care providers when using PrEP medications or any other prescription or over-the-counter medications.
TDF/FTC: Pregnancy is not a contraindication to the use of TDF/FTC as PrEP. Clinicians should counsel pregnant patients about the risks and benefits of continuing TDF/FTC for HIV prevention during pregnancy. Available data suggest that TDF/FTC as PrEP does not increase the risk of congenital anomalies (the use of ART medications during pregnancy is monitored through the Antiretroviral Pregnancy Registry). Conflicting results have been observed in studies of bone mineral density in infants born to women taking TDF as a component of ART for HIV Siberry, et al. 2015; Vigano, et al. 2011. One study suggested up to a 15% decrease in bone mineral density in infants exposed to TDF in utero compared with infants who were not exposed to TDF Siberry, et al. 2015, whereas another study found no association between in utero TDF exposure and infant bone mineral density Vigano, et al. 2011. In a study of pregnant women who did not have HIV in whom TDF was used as prophylaxis to prevent transmission of HBV, there was no difference in bone mineral density at 1 year in TDF-exposed infants compared with infants not exposed to TDF in utero Salvadori, et al. 2019.
Infant exposure to TDF/FTC through breast milk is much lower than TDF exposure in utero; evidence to date suggests that TDF is safe during breastfeeding Liotta, et al. 2016; Ehrhardt, et al. 2015. Longer-term follow-up studies of TDF-exposed infants are ongoing and will provide further information and guidance on the use of PrEP in this population Mugwanya, et al. 2016. Although data on breastfeeding effects are limited, TDF/FTC is commonly prescribed as part of an ART regimen before, during, and after pregnancy, and the benefit of preventing HIV infection and subsequent perinatal transmission among individuals at increased risk outweighs the theoretical concerns associated with prescribing TDF/FTC as PrEP during breastfeeding, pending further data.
CAB LA: There are insufficient data on the safety of CAB LA as PrEP in pregnancy, and CAB LA should be used during pregnancy only if the expected benefit justifies the potential risk to the fetus. In animal reproduction studies, parturition was delayed, and stillbirths and neonatal deaths increased when oral CAB was given at >28 times the recommended human dose FDA(a) 2021. More data should become available over time. In the interim, care providers should recommend that individuals planning to conceive or become pregnant while receiving CAB LA as PrEP switch to TDF/FTC if it is an appropriate option and they wish to continue PrEP.
Laboratory Testing Before PrEP Initiation
| RECOMMENDATION |
Laboratory Testing Before PrEP Initiation
|
Resource: Appendix: Care Provider Checklists for PrEP Initiation, Regimen Choice, and Follow-Up Abbreviations: Ag/Ab, antigen/antibody; ART, antiretroviral therapy; CAB LA, long-acting injectable cabotegravir (brand name Apretude); CEI, Clinical Education Initiative; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis. |
HIV Testing
Risk assessment: Before starting PrEP, all patients should be evaluated for possible HIV infection and assessed for acute HIV. The goal of risk assessment is to identify potential HIV exposures in the prior 4 weeks and flu- or mono-like symptoms in the previous 6 weeks.
Both an HIV-1/2 Ag/Ab combination immunoassay and an HIV RNA test should be performed within 1 week before PrEP is started. HIV RNA (viral load) testing is performed at baseline to rule out acute HIV infection regardless of reported risk, as individuals may be reluctant to disclose a recent potential risk exposure. If a confirmed negative result is not available at the time of the patient’s initial visit, a rapid HIV-1/2 test should be performed for same-day PrEP initiation.
Drug-resistant virus has been found in patients with undiagnosed HIV who initiated PrEP with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC; brand name Truvada), tenofovir alafenamide/emtricitabine (TAF/FTC; brand name Descovy), or CAB LA Landovitz, et al. 2021; Cox, et al. 2020; Molina, et al. 2022; Lehman, et al. 2015.
Same-day initiation of oral PrEP: Once laboratory specimens are obtained (see Table 3, below), PrEP may be initiated while test results are pending if the patient has not had symptoms or signs of acute HIV in the prior 6 weeks, has no history of renal disease, and no risk exposures in the past 72 hours requiring PEP, as long as laboratory results will be available and addressed within 7 days and a rapid HIV test result is negative. In HIV care, the rapid start of treatment has been shown to better engage patients in care Ford, et al. 2018. Same-day initiation of PrEP has been shown to be safe, and delayed initiation of PrEP has been associated with a significant rate of loss to follow-up Kamis, et al. 2019; Mikati, et al. 2019. Same-day PrEP initiation may engage patients more fully in care and reduce exposures to HIV while test results are pending and encourages immediate attention to insurance coverage for PrEP or identification of other options for payment if needed.
Same-day PrEP initiation risks starting a nonsuppressive ART regimen in someone with HIV. However, if laboratory results are available promptly, then the PrEP regimen for an individual who tests positive for HIV can be intensified to a fully suppressive ART regimen, and a referral for HIV care can be made. If the baseline HIV testing result is positive after CAB is initiated, the care provider should consult with an experienced HIV care provider regarding the best way to intensify the PrEP regimen to a fully suppressive ART regimen (see guideline section Managing a Positive HIV Test Result). TAF/FTC may require a prior authorization, which makes same-day initiation a challenge, but generic TDF/FTC is usually available for same-day initiation.
Same-day initiation of an oral CAB lead-in or CAB LA: If prior insurance authorization is required, same-day CAB initiation (oral or injection) may not be an option. Implementation challenges such as stocking and storing medications in advance may also be prohibitive. If same-day CAB initiation is not an option, prescription of an oral regimen should be discussed as an interim option while barriers to accessing CAB LA injections are addressed.
Although delays such as insurance barriers may impede initiation of PrEP, the overall goal should be same-day initiation in patients without renal disease, need for PEP, or signs or symptoms of acute HIV.
| KEY POINT |
|
Initiating PrEP during the HIV testing window period: The “window period” is the time between when an individual has acquired HIV and when a diagnostic test can detect infection. The median time to positivity is 12 days for an HIV viral load test and 18 days for a laboratory-based HIV-1/2 Ag/Ab combination immunoassay; however, the 99th percentile for a positive test is 33 days for an HIV viral load test and 42 days for a laboratory-based HIV-1/2 Ag/Ab combination immunoassay Delaney, et al. 2017. Clinicians should not defer initiation of PrEP in candidates who, based on their reported sexual and drug use exposures, may be in the window period for seroconversion; doing so risks additional exposures and significant delays in PrEP initiation. Repeat HIV testing 1 month after initiation to help identify potentially positive individuals in a timely manner (see guideline section Ongoing Laboratory Testing).
Recommended Laboratory Testing
Table 3, below, lists the baseline laboratory tests that should be performed at the pre-prescription visit for individuals who will initiate PrEP. When an individual is engaged in care to receive PrEP, primary healthcare may be offered as indicated, including vaccinations against hepatitis A and B viruses, human papillomavirus, meningococcus, influenza, and COVID-19.
For more information, see Centers for Disease Control and Prevention Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2022 and COVID-19 ACIP Vaccine Recommendations and NYSDOH Health Advisory: NYSDOH Meningococcal Vaccine Recommendations for HIV-Infected Individuals and Those at High Risk of HIV Infection.
| Abbreviations: Ab, antibody; Ag, antigen; anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; CAB LA, long-acting injectable cabotegravir (brand name Apretude); CDC, Centers for Disease Control and Prevention; CrCl, creatinine clearance; HAV, hepatitis A virus; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; IgG, immunoglobulin G; MSM, men who have sex with men; NAAT, nucleic acid amplification test; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (brand name Truvada); UCSF, University of California San Francisco.
Notes:
|
||
| Table 3: Recommended Laboratory Tests for All Patients Within 1 Week Before Initiating PrEP [a] | ||
| Purpose (rating) | Test | Comments |
| HIV status (A*) |
|
|
| Renal function (A*) | Serum creatinine and calculated CrCl |
|
| Pregnancy status (A3) | Pregnancy test for all individuals of childbearing potential |
|
| HBV infection status (A2†) | HBV serologies: HBsAg, anti-HBs, and anti-HBc (IgG or total) |
|
| Syphilis screening (A2†) | All patients: Syphilis testing [d] | Screen for syphilis according to the laboratory’s testing algorithm [d] |
| Gonorrhea and chlamydia screening (A2†) |
|
|
| HCV infection status (A3) | HCV serology with reflex to RNA | Inform patients with HCV about transmission risk and offer or refer for treatment [f] |
| HAV infection status (good practice) | HAV serology for MSM and individuals at high risk for HAV infection; see footnote [g] | Vaccinate nonimmune patients |
| Hepatic function (good practice) | Serum liver enzymes | Increased serum liver enzymes may indicate acute or chronic viral hepatitis infection and require further evaluation |
| Assess for preexisting renal disease, proteinuria, and glycosuria (good practice) | Urinalysis | Only calculated CrCl is used to guide decisions regarding the use of TDF/FTC and TAF/FTC as PrEP based on renal function |
| SELECTED GOOD PRACTICE REMINDERS |
Follow-up after PrEP initiation:
At each visit [b]:
Risk reduction:
|
|
Notes:
|
Ongoing Laboratory Testing
| RECOMMENDATION |
HIV Testing
Renal Function Testing
STI Screening
HCV Screening
Pregnancy Screening
|
Resource: Appendix: Care Provider Checklists for PrEP Initiation, Regimen Choice, and Follow-Up Abbreviations: Ag/Ab, antigen/antibody; CAB LA, long-acting injectable cabotegravir (brand name Apretude); CrCl, creatinine clearance; HCV, hepatitis C virus; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (brand name Truvada). |
Services for follow-up and monitoring of patients receiving PrEP are part of a comprehensive HIV prevention plan: routine HIV testing; risk-reduction counseling; access to condoms and syringes; STI, mental health, and substance use screening; and referral for treatment when indicated.
Table 4, below, lists the laboratory tests that should be performed for individuals using oral or injectable PrEP.
| Abbreviations: Ab, antibody; Ag, antigen; CAB, cabotegravir (brand name Vocabria); CAB LA, long-acting cabotegravir (brand name Apretude); CrCl, creatinine clearance; HCV, hepatitis C virus; MSM, men who have sex with men; NAAT, nucleic acid amplification test; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (brand name Truvada).
Notes:
|
|||
| Table 4: Recommended Routine Laboratory Testing for Patients Taking PrEP | |||
| Test | Laboratory Testing Indications | ||
| All PrEP Regimens | Oral PrEP With TDF/FTC or TAF/FTC | Injectable PrEP With CAB LA | |
| HIV-1/2 Ag/Ab combination immunoassay [a] |
|
|
|
| HIV RNA assay [a] | When a patient has symptoms of acute HIV [b] (A2) |
|
|
| Serum creatinine and calculated CrCl | — |
|
At least annually (A3) |
| Syphilis screening (A2†)
Note: Screening can be less frequent in those at lower risk |
|
Every 3 months | Every 2 to 4 months based on reported ris |
| Gonorrhea and chlamydia screening (A2†)
Note: Screening can be less frequent in those at lower risk |
|
Every 3 months | Every 2 to 4 months based on reported risk |
| HCV serology [d] | At least annually if at risk (A3) | — | — |
| Pregnancy test in patients of childbearing potential |
|
— | — |
| Urinalysis | N/A | Annually (B3) | N/A |
Download Table 4: Recommended Routine Laboratory Testing for Patients Taking PrEP Printable PDF
HIV Testing
For individuals taking PrEP, routine HIV testing is recommended for early detection of PrEP failure. None of the available regimens are adequate for treating acute or chronic HIV infection. Continued use of only TDF/FTC, TAF/FTC, or CAB LA in the presence of HIV may lead to viral resistance to these drugs.
Routine HIV testing with oral PrEP: A quarterly (every 3 months) HIV-1/2 Ag/Ab combination immunoassay is recommended for individuals taking TDF/FTC or TAF/FTC as PrEP. Quarterly HIV RNA testing is not needed for patients who are stable on oral PrEP.
Breakthrough HIV infections among individuals adherent to oral PrEP are rare. When failure of an oral PrEP regimen (TDF/FTC or TAF/FTC) occurs, it is usually associated with poor adherence and is infrequently associated with the development of resistance. When drug resistance does develop, it is typically an M184V/I resistance mutation, which has little or no effect on subsequent response to HIV treatment. The development of the K65R resistance mutation (associated with tenofovir) has rarely been reported van de Vijver, et al. 2021; Gibas, et al. 2019. Unrecognized acute HIV infection at the time of PrEP initiation carries the highest risk for developing resistance mutations van de Vijver, et al. 2021; Gibas, et al. 2019. For this reason, this committee recommends that clinicians perform baseline HIV RNA testing for all patients before initiating PrEP.
In the HPTN 083 study, there was less delay in detection of new HIV infections with seroconversion for participants receiving TDF/FTC than for those receiving CAB LA. There were a median of 34 days and 31 days of unrecognized baseline and incident HIV infections, respectively, in the TDF/FTC arm, compared with 62 days and 98 days, respectively, in the CAB LA arm Marzinke(a), et al. 2021.
HIV RNA testing is recommended for individuals who report an interruption in PrEP of more than 1 week or report missing doses of PrEP during a time of sexual activity since the preceding visit.
| KEY POINTS |
|
Routine HIV RNA testing is not recommended for individuals stable on TDF/FTC or TAF/FTC as PrEP for the following reasons:
|
Testing for HIV every 3 months is based on good practice rather than evidence. If HIV testing is missed, every effort should be made to avoid interruption of PrEP, as the potential harm of discontinuing PrEP outweighs the theoretical risk of delaying HIV testing in a stable patient. It is reasonable to provide an additional month of PrEP and plan for HIV testing as soon as possible.
In-office visits are not a requirement for ongoing prescription of PrEP. It is reasonable for patients established on oral PrEP to be tested for HIV every 3 months, either on-site or at an outside laboratory, and to see their care providers every 6 to 12 months. Requiring in-office visits may create a barrier to PrEP access for some individuals. Alternative monitoring models, such as at-home testing and telehealth visits, can decrease PrEP access and monitoring barriers.
Routine HIV testing with CAB LA: CAB LA injections are administered at in-office visits every 2 months; there are currently no alternative administration models. HIV testing should be performed at the end of the oral CAB lead-in (if implemented) and at every injection visit. Breakthrough HIV infections were observed in 4 individuals who received on-time CAB LA injections in the HPTN 083 study, and were also observed during the oral CAB lead-in phase and during treatment interruptions Marzinke(a), et al. 2021. Adherence to the CAB LA injection schedule is not a guarantee of protection against HIV acquisition.
HIV testing should include an HIV-1/2 Ag/Ab combination immunoassay and HIV RNA test using FDA-approved tests and sample types (plasma or serum). Studies have reported atypical test results with missed diagnoses when the standard Centers for Disease Control and Prevention/American Public Health Laboratories Laboratory Testing Algorithm in Serum/Plasma was used with individuals on antiretroviral therapy (ART) at the time of HIV seroconversion. In the HPTN 083 study, there was a median delay of 62 days from the time of infection to HIV diagnosis for unrecognized baseline infections in individuals on CAB LA, and of 98 days for incident infections, with the subsequent development of integrase resistance mutations in 5 individuals Eshleman, et al. 2022; Marzinke(a), et al. 2021.
Because there may be delays in HIV-1/2 Ag/Ab combination immunoassay positivity and indeterminate or negative results for supplemental HIV-1/HIV-2 Ab differentiation tests, concomitant routine HIV RNA testing can improve timely detection of breakthrough HIV infection.
ART can blunt viral load response. In one study, qualitative RNA testing was the most reliable detection method in cases with retrospective testing; however, the sensitivity of newer quantitative viral load tests is equal to available qualitative RNA tests. Marzinke(a), et al. 2021.
HIV testing if exposure is suspected: For patients who present for PrEP initiation but have had a potential HIV exposure within the past 30 days, initial HIV testing may not detect early infection. Therefore, repeat HIV testing 1 month after initiation is recommended to rule out early HIV infection. For patients who report an interruption in PrEP during a time of sexual activity, an HIV-1/2 Ag/Ab combination immunoassay and HIV RNA test are recommended when reinitiating PrEP.
HIV testing if acute HIV is suspected: Vigilance for signs and symptoms of potential HIV seroconversion in individuals taking PrEP is crucial. If acute HIV is suspected, the clinician should perform an HIV-1/2 Ag/Ab combination immunoassay and a quantitative HIV RNA test Chin, et al. 2013; Apoola, et al. 2002. When testing for acute HIV infection is indicated, any use of a rapid HIV-1/2 Ag/Ab test should be followed with a laboratory-based HIV-1/2 Ag/Ab combination immunoassay. Detection of HIV RNA or Ag in the absence of serologic evidence of HIV should be considered a preliminary positive result (see guideline section Managing a Positive HIV Test Result).
For more detailed recommendations on testing for acute HIV, see the NYSDOH AI guidelines Diagnosis and Management of Acute HIV Infection and HIV Testing.
| KEY POINTS |
|
Routine Laboratory Testing
Renal function testing: One sign of tenofovir toxicity is the development of proteinuria. A baseline urinalysis helps to identify preexisting proteinuria before initiating PrEP. Periodic renal function monitoring is also important while a patient is taking TDF/FTC or TAF/FTC as PrEP. An elevated creatinine level should prompt an assessment for causes of renal dysfunction other than tenofovir, such as the use of nonsteroidal anti-inflammatory drugs, and an assessment for possible spurious elevation caused by the use of creatine supplements.
More frequent creatinine screening may be appropriate in individuals >40 years old because renal toxicity is more likely to occur in this population Gandhi, et al. 2016. More frequent screening may also be appropriate for patients with comorbidities, such as diabetes or hypertension, and patients taking concomitant nephrotoxic drugs that might increase the risk of renal dysfunction.
CAB is not known to cause renal dysfunction, but caution and increased monitoring for adverse effects is recommended when CrCl is <30 mL/min. Baseline testing and annual monitoring of renal function are recommended.
STI screening: Many patients who elect to initiate PrEP are at high risk of acquiring STIs, including syphilis and gonorrheal and chlamydial infections. Initiating PrEP care for individuals at risk for HIV provides an opportunity for routine STI monitoring and treatment. One modeling study demonstrated that increased PrEP engagement along with routine STI screening and treatment would lower STI rates through detection and treatment of asymptomatic STIs that might otherwise remain undiagnosed Jenness, et al. 2017.
Data from New York State and other jurisdictions show high rates of STIs, a majority of which are asymptomatic, making an inquiry about symptoms insufficient, and supporting testing for syphilis and gonococcal and chlamydial infections at frequent intervals Tang, et al. 2020; Golub, et al. 2016; Liu, et al. 2016. Less frequent testing can be considered for individuals at lower risk of acquiring STIs. However, frequent testing leads to early diagnosis and treatment of asymptomatic STIs and can decrease the development of complicated infections, such as neurosyphilis or pelvic inflammatory disease, and decrease transmission to sex partners.
STI testing is recommended every 3 months for individuals taking oral PrEP. Individuals receiving CAB LA as PrEP require visits every 2 months; STI screening can be performed at each injection visit for those at highest risk or every 4 months for those with average risk. The frequency of STI screening in all patients on PrEP can be adjusted based on risk assessment and occur less often in those at low risk for STI acquisition.
Routine syphilis serologic testing should be performed for all patients taking PrEP, and all patients should be asked about anatomic sites of potential exposure and screened for gonococcal and chlamydial infections accordingly. Men who have sex with men (MSM) and transgender women have particularly high rates of extragenital STIs, and performing only urine-based screening misses a majority of infections Pitasi, et al. 2019; Patton, et al. 2014. Three-site testing (genital, pharyngeal, and rectal) for gonococcal and chlamydial infections should be the default testing plan for MSM and transgender women unless such testing is declined because of lack of exposure at a site. A recent study reported high rates of extragenital gonococcal and chlamydial infections in transgender men attending STI clinics Pitasi, et al. 2019; therefore, risk for extragenital STIs should be considered in this population as well.
Because nucleic acid amplification testing has much higher sensitivity than culture, it is preferred for the diagnosis of gonococcal or chlamydial infection. The U.S. Food and Drug Administration has approved diagnostic extragenital gonorrhea and chlamydia tests FDA(b) 2019, making access to testing more readily available.
Vaginal swabs are preferred over urine-based testing for cervical infections because of their sensitivity. For urethral infections, urine testing is preferred because of comfort. For transgender women with a neovagina, data are lacking regarding optimal specimen type (neovaginal swab vs. urine-based testing). There are some reports of gonococcal infections detected via testing obtained from the neovagina van der Sluis, et al. 2015. Self-obtained swabs for vaginal, rectal, and pharyngeal specimen types have performed well in many studies and are preferred by many patients (see STI self-collection outside of a clinic setting in NYS Question & Answer) Dize, et al. 2016; Lunny, et al. 2015; Workowski and Bolan 2015; Taylor, et al. 2013.
| KEY POINTS |
|
Annual HCV screening: An increased risk for HCV acquisition has been noted in MSM using PrEP Price, et al. 2019; Hoornenborg(b), et al. 2017. MSM with HIV Fierer and Factor 2015; Bradshaw, et al. 2013; Fierer 2010 and to a lesser degree MSM without HIV McFaul, et al. 2015 are at increased risk for acute HCV. A case-control study of MSM with HIV found that the presence of recent ulcerative STIs or risk behaviors, such as unprotected receptive anal intercourse, sharing of sex toys, unprotected fisting, injecting drugs, and sharing straws when snorting drugs, contributed to an increased risk of HCV acquisition Vanhommerig, et al. 2015. These may also be important risk factors in the general population. New elevations in liver enzymes can be a sign of acute HCV. Annual screening for HCV in MSM, transgender women, and individuals using injection drugs who are using PrEP is recommended, with more frequent screening considered for those at highest risk or with any elevations of liver function tests.
Pregnancy screening: In individuals of childbearing potential taking PrEP, routine assessment for the possibility of pregnancy and testing as appropriate decrease potential concerns associated with unplanned pregnancies. Individuals of childbearing potential who are using PrEP and wish to avoid pregnancy should be offered contraception. TDF/FTC as PrEP does not affect levels of hormonal contraceptives, and CAB LA does not affect levels of oral contraceptives and is not expected to affect levels of long-acting contraception.
Managing a Positive HIV Test Result
| RECOMMENDATIONS |
Suspected Acute HIV
Asymptomatic Patients With a Reactive HIV Screening Test Result While Using PrEP
Ambiguous HIV Test ResultsTDF/FTC, TAF/FTC, or CAB LA used as PrEP may alter viral load and immune response and cause ambiguous HIV test results when following the current CDC/APHL HIV testing algorithm.
ART Selection for a Positive or Ambiguous HIV Test Result
|
Abbreviations: Ag/Ab, antigen/antibody; APHL, Association of Public Health Laboratories; ART, antiretroviral therapy; CAB LA, long-acting injectable cabotegravir (brand name Apretude); CDC, Centers for Disease Control and Prevention; CEI, Clinical Education Initiative; DRV/COBI, cobicistat-boosted darunavir (brand name Prezcobix); DTG, dolutegravir (brand name Tivicay); BIC, bictegravir; INSTI, integrase strand transfer inhibitor; PrEP, pre-exposure prophylaxis; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TAF/FTC/BIC, tenofovir alafenamide/emtricitabine/bictegravir (brand name Biktarvy); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (brand name Truvada). |
Suspected Acute HIV
Vigilance for signs and symptoms of potential HIV seroconversion in patients receiving PrEP is crucial. TDF/FTC, TAF/FTC, or CAB LA as PrEP are not adequate treatments for acute or chronic HIV infection, and continued use in the presence of HIV may lead to the emergence of viral resistance to these drugs.
The mean time from HIV exposure to onset of symptoms is generally 2 to 4 weeks, with a range of 5 to 29 days; however, some patients are asymptomatic, and some have presented with symptoms up to 3 months after exposure Apoola, et al. 2002. This time course may be prolonged in patients who acquire HIV while on PrEP, and symptoms of acute HIV infection may be blunted by TDF/FTC, TAF/FTC, or CAB use. While evaluating patients for acute HIV, advise them to refrain from sexual activity or use condoms to minimize the risk of transmitting HIV to a partner without HIV.
For patients with symptoms consistent with acute HIV infection who have a nonreactive HIV test result but have a plasma HIV RNA level ≥200 copies/mL, a clinician can make a presumptive diagnosis of acute HIV, perform HIV genotype testing, obtain routine initial HIV laboratory tests, and recommend immediate initiation of ART.
For patients who have a nonreactive HIV test result but have an HIV RNA level <200 copies/mL, clinicians should repeat HIV RNA testing and repeat HIV diagnostic testing according to the standard HIV laboratory testing algorithm to exclude a false-positive test result versus a true-positive test result with a blunted viral response due to the presence of TDF/FTC, TAF/FTC, or CAB. Unless suspicion for acute HIV is low, initiate ART while a definitive diagnosis is sought.
Asymptomatic Patients With a Reactive HIV Screening Test Result
HIV acquisition in patients who are fully adherent to oral PrEP is rare. If a patient taking oral PrEP tests positive for HIV on a routine HIV-1/2 Ag/Ab combination immunoassay, determine if there has been any medication interruption or decreased adherence. A false-positive test result is more likely to occur when patients are not fully adherent to PrEP. There is an increased possibility of a true-positive test result if an individual reports gaps in adherence, medication access, or symptoms consistent with acute HIV in the period since their last HIV test. There are no commercially available tests of TDF concentrations to confirm longer-term adherence in someone with possible seroconversion on PrEP. Hair samples and dried blood spots are utilized as tests in research only. Because false-positive HIV-1/2 Ag/Ab combination immunoassay results do occur and there is a risk of HIV exposure if PrEP is discontinued, it is recommended that PrEP be continued. Clinicians should decide whether to continue PrEP or intensify to a fully suppressive ART regimen (see above) while awaiting confirmatory test results based on the degree of suspicion for a false-positive versus a true-positive HIV test result. Clinicians should perform supplemental diagnostic testing as soon as possible according to the standard HIV laboratory testing algorithm.
For asymptomatic patients with a positive HIV test result who are receiving CAB LA, assess for missed injections or adherence to the oral CAB during lead-in (if implemented). If PrEP has been interrupted, there is a much higher likelihood of a true-positive HIV test result. There were 4 breakthrough HIV infections with CAB LA despite on-time injections in the HPTN 083 study Landovitz, et al. 2021, although there were no breakthrough infections with on-time injections in the HPTN 084 study Delany-Moretlwe, et al. 2022. Therefore, adherence to the CAB LA injection schedule does not guarantee protection, although breakthrough infections are uncommon. If a patient is nonadherent to either oral or injectable CAB, the clinician should prescribe a non-INSTI-based ART regimen, as discussed above, while awaiting confirmation of HIV and resistance test results. If a patient has been receiving injections on time, the possibility of a false-positive HIV test is higher, and the clinician can choose whether to await confirmation or prescribe a non-INSTI-based ART regimen pending test results.
If supplemental laboratory testing confirms HIV infection, the clinician should perform quantitative HIV RNA testing (if not already obtained) to measure viral load, order ART initiation laboratory testing, perform genotypic resistance testing, and initiate ART as in ART Selection For Patients Who Acquire HIV While on PrEP, below.
| KEY POINTS |
|
Ambiguous HIV Test Results
The use of TDF/FTC, TAF/FTC, or CAB LA as PrEP may alter viral load and immune response and cause ambiguous HIV test results when the current CDC/APHL HIV testing algorithm is followed Zucker, et al. 2018; Hoornenborg(a), et al. 2017; Knox, et al. 2017; Markowitz, et al. 2017. Viral load may be suppressed. An HIV-1/2 Ag/Ab combination immunoassay may be reactive, but supplemental testing (HIV-1/HIV-2 Ab differentiation, HIV RNA, or Western blot) may not consistently be reactive, or the time to reactivity may be delayed. In the HPTN 083 study of CAB LA as PrEP, in which there were 12 incident HIV infections, the use of HIV Ag/Ab testing alone led to a significant delay in detection of HIV infection, and retrospective analysis found ambiguous test results Marzinke(a), et al. 2021.
In cases of ambiguous HIV test results in individuals taking PrEP, repeat HIV testing in a few days may resolve ambiguity if the initial results were due to early infection or technical issues.
If ambiguity persists, the options are to continue PrEP, intensify to a full HIV treatment regimen while waiting for additional testing to resolve the ambiguity, or discontinue PrEP and allow viral replication to occur and be measured if the patient does have HIV. However, discontinuing PrEP leaves an uninfected individual at risk. Delaying intensification risks loss of the theoretical virologic and immunologic benefits of early ART in an individual who does have HIV. Given the complexities of this issue, consultation with an experienced HIV care provider is recommended (see A Strategy for PrEP Clinicians to Manage Ambiguous HIV Test Results During Follow-Up Visits for a thorough review of this topic Smith, et al. 2018).
ART Selection For Patients Who Acquire HIV While on PrEP
Because HIV acquisition in individuals taking oral PrEP is most often due to nonadherence, the majority of infections are found to be wild-type virus. When resistance does occur, FTC-associated resistance due to the emergence of the M184V/I mutation is commonly found; tenofovir-associated resistance with the K65R mutation is rare Girometti, et al. 2022; Mayer, et al. 2020; Lehman, et al. 2015. Among individuals receiving CAB LA in the HPTN 083 study, INSTI-associated mutations were observed in 1 individual with baseline HIV infection and 4 individuals with incident HIV infection Landovitz, et al. 2021. No participants in the HPTN 084 study developed INSTI resistance Marzinke(b), et al. 2021. There are no definitive protocols for specific antiretroviral agents to use in the case of suspected seroconversion in individuals taking PrEP. However, for patients taking oral PrEP, given the rarity of tenofovir-associated mutations during PrEP use, a reasonable option would be to continue TDF/FTC or TAF/FTC and add a third agent with a high resistance barrier, such as DRV/COBI, DTG, or BIC (available as TAF/FTC/BIC) while awaiting HIV confirmation and resistance test results. For patients receiving CAB LA, initiation of a non-INSTI-based ART regimen is recommended while awaiting confirmation of HIV infection and resistance test results. If no INSTI resistance is found, the patient can be switched to an INSTI-based ART regimen if desired. For more information, see the NYSDOH AI guidelines Rapid ART Initiation and Selecting an Initial ART Regimen.
Discontinuing PrEP
| RECOMMENDATIONS |
Discontinuing PrEP
|
Abbreviations: CAB LA, long-acting cabotegravir injectable (brand name Apretude); CrCl, creatinine clearance; HBV, hepatitis B virus; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (brand name Truvada). |
The 2-drug PrEP regimens of TDF/FTC and TAF/FTC and the single-drug regimen of CAB LA are not adequate as HIV antiretroviral therapy (ART). If HIV infection is confirmed, PrEP should be converted immediately to a fully suppressive HIV ART regimen (see guideline section Managing a Positive HIV Test Result).
Renal function should be monitored as outlined in the guideline section Ongoing Laboratory Testing. If an increase in serum creatinine or a decrease in calculated CrCl is observed, evaluate potential causes other than TDF or TAF use, such as the use of nonsteroidal anti-inflammatory drugs, spurious elevations due to creatine supplementation, or recent heavy exercise leading to muscle breakdown, and repeat testing to confirm the finding before considering discontinuation. To decrease drug exposure, on-demand TDF/FTC dosing can be considered for MSM with borderline renal function.
TDF/FTC as PrEP should be discontinued in patients who develop a confirmed CrCl <50 mL/min to avoid further toxicity. Although TDF/FTC for treatment of HIV can be adjusted to every-other-day dosing in patients with a CrCl between 30 mL/min and 49 mL/min, this strategy has not been established for PrEP and should not be used. TAF/FTC is an option for MSM and transgender women with CrCl ≥30 mL/min. CAB LA as PrEP is an option for all individuals with renal dysfunction who are at risk for HIV infection via sexual exposure. Increased monitoring for adverse effects is recommended for those with severe or end-stage renal impairment.
Individuals may choose to discontinue PrEP when their risk for HIV acquisition is no longer a concern. If an individual decides to stop using PrEP because of the challenges of adhering to medication and visits but is still at risk for HIV acquisition, make attempts to individualize care to maintain access to the protection afforded by PrEP. For individuals who do not adhere to the testing requirements for the safe prescribing of PrEP, PrEP should be discontinued only after repeated attempts have been made to accommodate specific patient needs and engage the patient in ongoing PrEP care.
Because discontinuation of TDF/FTC or TAF/FTC in patients with chronic, active HBV can exacerbate HBV Buti, et al. 2015; Dore, et al. 2010; Chamorro, et al. 2005, an alternative treatment plan for these individuals is critical. For more information, see CDC Recommendations for Routine Testing and Follow-up for Chronic Hepatitis B Virus Infection.
| KEY POINTS |
|
Appendix: Care Provider Checklists for PrEP Initiation, Regimen Choice, and Follow-Up
Checklist 1: PrEP Initiation
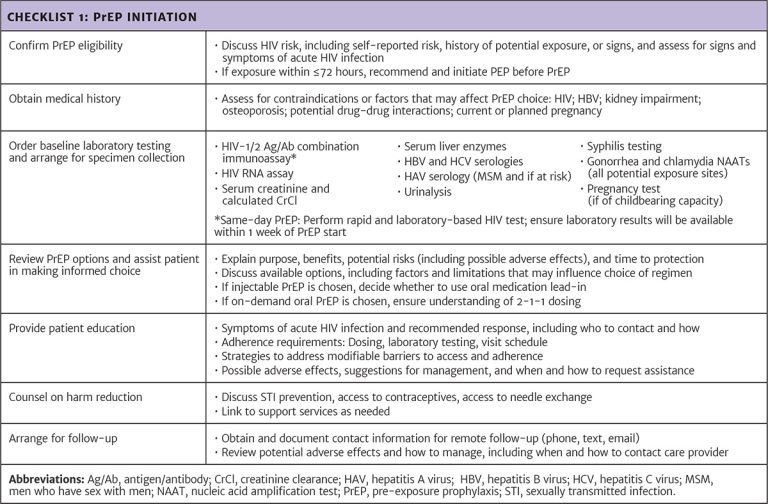
Download checklist: PrEP Initiation
Checklist 2: Key Factors in Choice of PrEP Regimen
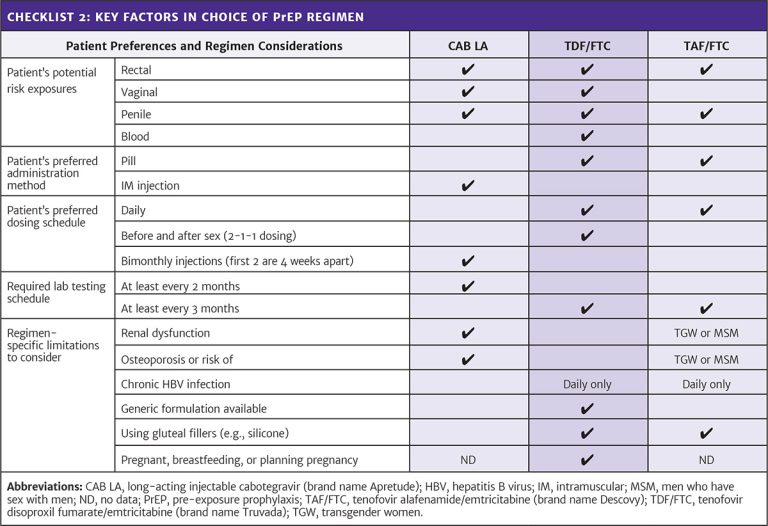
Download checklist: Key Factors in Choice of PrEP Regimen
Checklist 3: PrEP Follow-up
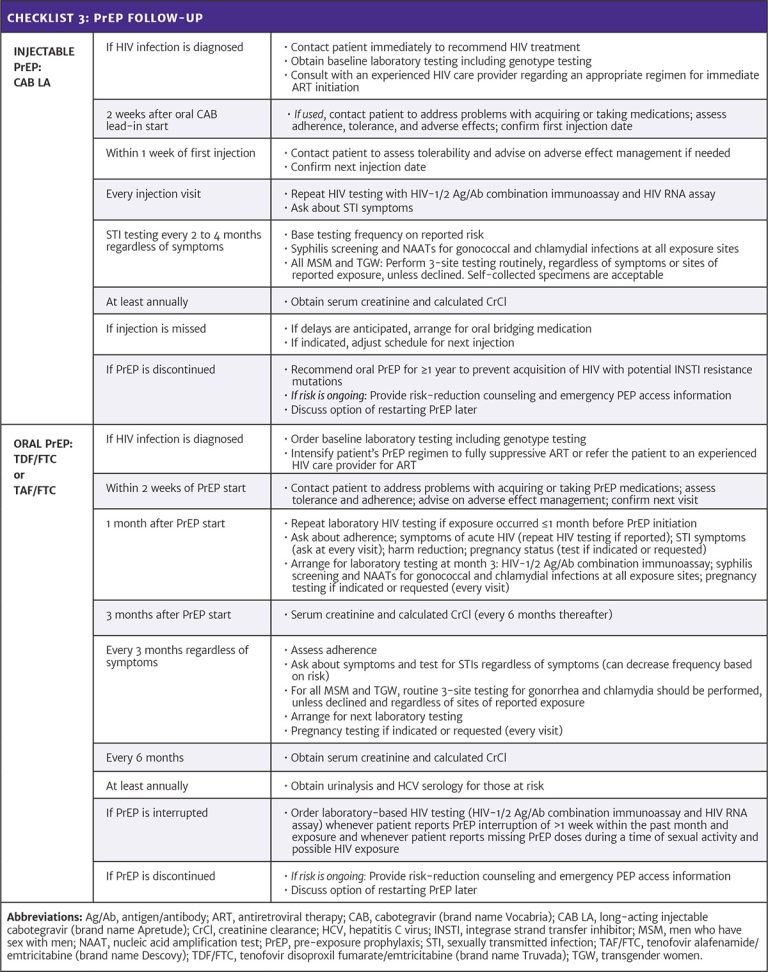
All Recommendations
| ALL RECOMMENDATIONS: PREP TO PREVENT HIV AND PROMOTE SEXUAL HEALTH |
Indications
Contraindications to PrEP
Choice of Regimen
TDF/FTC
TAF/FTC
Patients With HBV Infection
CAB LA
Laboratory Testing Before PrEP Initiation
HIV Testing
Renal Function Testing
STI Screening
HCV Screening
Pregnancy Screening
Suspected Acute HIV
Asymptomatic Patients With a Reactive HIV Screening Test Result While Using PrEP
Ambiguous HIV Test ResultsTDF/FTC, TAF/FTC, or CAB LA used as PrEP may alter viral load and immune response and cause ambiguous HIV test results when following the current CDC/APHL HIV testing algorithm.
ART Selection for a Positive or Ambiguous HIV Test Result
Discontinuing PrEP
|
Resource: Appendix: Care Provider Checklists for PrEP Initiation, Regimen Choice, and Follow-Up Abbreviations: Ag/Ab, antigen/antibody; APHL, Association of Public Health Laboratories; ART, antiretroviral therapy; BIC, bictegravir; CAB LA, long-acting injectable cabotegravir (brand name Apretude); CDC, Centers for Disease Control and Prevention; CEI, Clinical Education Initiative; CrCl, creatinine clearance; DRV/COBI, cobicistat-boosted darunavir (brand name Prezcobix); DTG, dolutegravir (brand name Tivicay); HBV, hepatitis B virus; HCV, hepatitis C virus; IM, intramuscular; INSTI, integrase strand transfer inhibitor; MSM, men who have sex with men; nPEP, non-occupational post-exposure prophylaxis; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection; TAF/FTC, tenofovir alafenamide/emtricitabine (brand name Descovy); TAF/FTC/BIC, tenofovir alafenamide/emtricitabine/bictegravir (brand name Biktarvy); TDF/FTC; tenofovir disoproxil fumarate/emtricitabine (brand name Truvada). |
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making

Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Abdul-Quader A. S., Feelemyer J., Modi S., et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav 2013;17(9):2878-92. [PMID: 23975473]
Anderson P. L., Garcia-Lerma J. G., Heneine W. Nondaily preexposure prophylaxis for HIV prevention. Curr Opin HIV AIDS 2016;11(1):94-101. [PMID: 26633641]
Anderson P. L., Glidden D. V., Liu A., et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012;4(151):151ra125. [PMID: 22972843]
Antoni G., Tremblay C., Delaugerre C., et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV 2020;7(2):e113-20. [PMID: 31784343]
Apoola A., Ahmad S., Radcliffe K. Primary HIV infection. Int J STD AIDS 2002;13(2):71-78. [PMID: 11839160]
AVAC. Webinar: Time to protection for PrEP. 2017 Feb 9. https://www.avac.org/event/webinar-time-protection-prep [accessed 2022 Mar 17]
Baeten J. M., Donnell D., Ndase P., et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367(5):399-410. [PMID: 22784037]
Baeten J. M., Heffron R., Kidoguchi L., et al. Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1-serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med 2016;13(8):e1002099. [PMID: 27552090]
Bazzi A. R., Biancarelli D. L., Childs E., et al. Limited knowledge and mixed interest in pre-exposure prophylaxis for HIV prevention among people who inject drugs. AIDS Patient Care STDS 2018;32(12):529-37. [PMID: 30311777]
BHIVA. BHIVA/BASHH guidelines on the use of HIV pre-exposure prophylaxis (PrEP) 2018. 2019 Mar 4. https://www.bhiva.org/PrEP-guidelines [accessed 2022 Mar 17]
Biello K. B., Bazzi A. R., Mimiaga M. J., et al. Perspectives on HIV pre-exposure prophylaxis (PrEP) utilization and related intervention needs among people who inject drugs. Harm Reduct J 2018;15(1):55. [PMID: 30419926]
Bien C. H., Patel V. V., Blackstock O. J., et al. Reaching key populations: PrEP uptake in an urban health care system in the Bronx, New York. AIDS Behav 2017;21(5):1309-14. [PMID: 28025734]
Blackstock O. J., Moore B. A., Berkenblit G. V., et al. A cross-sectional online survey of HIV pre-exposure prophylaxis adoption among primary care physicians. J Gen Intern Med 2017;32(1):62-70. [PMID: 27778215]
Blair C. S., Li S., Chau G., et al. Brief report: hormonal contraception use and cabotegravir pharmacokinetics in HIV-uninfected women enrolled in HPTN 077. J Acquir Immune Defic Syndr 2020;85(1):93-97. [PMID: 32452972]
Blashill A. J., Ehlinger P. P., Mayer K. H., et al. Optimizing adherence to preexposure and postexposure prophylaxis: the need for an integrated biobehavioral approach. Clin Infect Dis 2015;60 Suppl 3:S187-90. [PMID: 25972502]
Blumenthal J., Goyal R., Burke L., et al. The bidirectional effects of hormone therapy and PrEP in transgender individuals. CROI; 2022 Feb 12-16. https://www.croiconference.org/abstract/the-bidirectional-effects-of-hormone-therapy-and-prep-in-transgender-individuals/
Blumenthal J., Jain S., He F., et al. Results from a pre-exposure prophylaxis demonstration project for at-risk cisgender women in the United States. Clin Infect Dis 2021;73(7):1149-56. [PMID: 33864370]
Bradshaw D., Matthews G., Danta M. Sexually transmitted hepatitis C infection: the new epidemic in MSM?. Curr Opin Infect Dis 2013;26(1):66-72. [PMID: 23242342]
Bramson H., Des Jarlais D. C., Arasteh K., et al. State laws, syringe exchange, and HIV among persons who inject drugs in the United States: history and effectiveness. J Public Health Policy 2015;36(2):212-30. [PMID: 25590514]
Buchacz K., McFarland W., Kellogg T. A., et al. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS 2005;19(13):1423-24. [PMID: 16103774]
Bujan L., Pasquier C. People living with HIV and procreation: 30 years of progress from prohibition to freedom?. Hum Reprod 2016;31(5):918-25. [PMID: 26975324]
Buti M., Casillas R., Riveiro-Barciela M., et al. Tenofovir discontinuation after long-term viral suppression in HBeAg negative chronic hepatitis B. Can HBsAg levels be useful?. J Clin Virol 2015;68:61-68. [PMID: 26071338]
Calabrese S. K., Earnshaw V. A., Underhill K., et al. The impact of patient race on clinical decisions related to prescribing HIV pre-exposure prophylaxis (PrEP): assumptions about sexual risk compensation and implications for access. AIDS Behav 2014;18(2):226-40. [PMID: 24366572]
Calabrese S. K., Earnshaw V. A., Underhill K., et al. Prevention paradox: medical students are less inclined to prescribe HIV pre-exposure prophylaxis for patients in highest need. J Int AIDS Soc 2018;21(6):e25147. [PMID: 29939488]
CDC(a). HIV prevention pill not reaching most Americans who could benefit – especially people of color. 2018 Mar 6. https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html [accessed 2022 Mar 17]
CDC(b). Preexposure prophylaxis for the prevention of HIV infection in the United States - 2017 update. 2018 Mar. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf [accessed 2022 Mar 14]
Celum C., Morrow R. A., Donnell D., et al. Daily oral tenofovir and emtricitabine-tenofovir preexposure prophylaxis reduces herpes simplex virus type 2 acquisition among heterosexual HIV-1-uninfected men and women: a subgroup analysis of a randomized trial. Ann Intern Med 2014;161(1):11-19. [PMID: 24979446]
Cespedes M., Majeed S. R., Prins M., et al. DISCOVER: no effect of hormones on F/TAF or F/TDF PK, efficacy & safety in transwomen. CROI; 2020 Mar 8-11. https://www.croiconference.org/abstract/discover-no-effect-of-hormones-on-f-taf-or-f-tdf-pk-efficacy-safety-in-transwomen/
Chamorro A. J., Casado J. L., Bellido D., et al. Reactivation of hepatitis B in an HIV-infected patient with antibodies against hepatitis B core antigen as the only serological marker. Eur J Clin Microbiol Infect Dis 2005;24(7):492-94. [PMID: 15990987]
Chan P. A., Mena L., Patel R., et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc 2016;19(1):20903. [PMID: 27302837]
Chin T., Hicks C., Samsa G., et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS 2013;27(7):392-97. [PMID: 23802143]
Choopanya K., Martin M., Suntharasamai P., et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381(9883):2083-90. [PMID: 23769234]
Cohen M. S., Chen Y. Q., McCauley M., et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493-505. [PMID: 21767103]
Cohen S. E., Sachdev D., Lee S. A., et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV 2019;6(1):e43-50. [PMID: 30503324]
Cottrell M. L., Garrett K. L., Prince H. M. A., et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J Antimicrob Chemother 2017;72(6):1731-40. [PMID: 28369415]
Cottrell M. L., Prince H. M. A., Schauer A. P., et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for HIV pre-exposure prophylaxis (PrEP). Clin Infect Dis 2019;69(12):2201-4. [PMID: 30963179]
Cottrell M. L., Yang K. H., Prince H. M., et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016;214(1):55-64. [PMID: 26917574]
Cox S., Parikh U. M., Heaps A. L., et al. Deep sequencing with unique molecular identifiers for evaluation of HIV-1 drug resistance in the DISCOVER pre-exposure prophylaxis trial. Abstract PDB0404. J Int AIDS Soc 2020;23(S4):118. http://programme.aids2020.org/Abstract/Abstract/5892
D'Angelo A. B., Davis Ewart L. N., Koken J., et al. Barriers and facilitators to pre-exposure prophylaxis uptake among Black women: a qualitative analysis guided by a socioecological model. J Assoc Nurses AIDS Care 2021;32(4):481-94. [PMID: 34171885]
Daughtridge G. W., Conyngham S. C., Ramirez N., et al. I am men's health: generating adherence to HIV pre-exposure prophylaxis (PrEP) in young men of color who have sex with men. J Int Assoc Provid AIDS Care 2015;14(2):103-7. [PMID: 25331226]
Delaney K. P., Hanson D. L., Masciotra S., et al. Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clin Infect Dis 2017;64(1):53-59. [PMID: 27737954]
Delany-Moretlwe S., Hughes J. P., Bock P., et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022;399(10337):1779-89. [PMID: 35378077]
Deutsch M. B., Glidden D. V., Sevelius J., et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015;2(12):e512-19. [PMID: 26614965]
Dize L., Barnes P., Barnes M., et al. Performance of self-collected penile-meatal swabs compared to clinician-collected urethral swabs for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium by nucleic acid amplification assays. Diagn Microbiol Infect Dis 2016;86(2):131-35. [PMID: 27497595]
Dore G. J., Soriano V., Rockstroh J., et al. Frequent hepatitis B virus rebound among HIV-HBV-coinfected patients following antiretroviral therapy interruption in the SMART study. AIDS 2010;24(6):857-65. [PMID: 20216301]
Drake A. L., Wagner A., Richardson B., et al. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014;11(2):e1001608. [PMID: 24586123]
Edelman E. J., Moore B. A., Calabrese S. K., et al. Primary care physicians' willingness to prescribe HIV pre-exposure prophylaxis for people who inject drugs. AIDS Behav 2017;21(4):1025-33. [PMID: 27896552]
Ehrhardt S., Xie C., Guo N., et al. Breastfeeding while taking lamivudine or tenofovir disoproxil fumarate: a review of the evidence. Clin Infect Dis 2015;60(2):275-78. [PMID: 25313254]
Eshleman S. H., Fogel J. M., Piwowar-Manning E., et al. Characterization of human immunodeficiency virus (HIV) infections in women who received injectable cabotegravir or tenofovir disoproxil fumarate/emtricitabine for HIV prevention: HPTN 084. J Infect Dis 2022;225(10):1741-49. [PMID: 35301540]
ETE Dashboard. PrEP use in New York State. 2020. https://etedashboardny.org/ [accessed 2022 Mar 14]
FDA. Truvada package insert. 2016 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021752s047lbl.pdf [accessed 2022 Mar 17]
FDA. Supplement approval for Truvada. 2018 May 15. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/021752Orig1s055ltr.pdf [accessed 2022 Mar 14]
FDA(a). FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic. 2019 Oct 3. https://www.fda.gov/news-events/press-announcements/fda-approves-second-drug-prevent-hiv-infection-part-ongoing-efforts-end-hiv-epidemic [accessed 2022 Mar 14]
FDA(a). Apretude package insert. 2021 Dec. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf [accessed 2022 Mar 17]
FDA(b). FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. 2019 May 23. https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea [accessed 2022 Mar 17]
FDA(b). FDA approves first injectable treatment for HIV pre-exposure prevention. 2021 Dec 20. https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention [accessed 2022 Mar 14]
Fierer D. S. Epidemic of sexually transmitted hepatitis C virus infection among HIV-infected men. Curr Infect Dis Rep 2010;12(2):118-25. [PMID: 21308508]
Fierer D. S., Factor S. H. Defining the scope of sexually transmitted hepatitis C virus epidemic among HIV-infected men who have sex with men in New York City. Sex Transm Dis 2015;42(7):400-401. [PMID: 26222756]
Ford N., Migone C., Calmy A., et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018;32(1):17-23. [PMID: 29112073]
Gandhi M., Glidden D. V., Mayer K., et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 2016;3(11):e521-28. [PMID: 27658870]
Garnett M., Hirsch-Moverman Y., Franks J., et al. Limited awareness of pre-exposure prophylaxis among black men who have sex with men and transgender women in New York city. AIDS Care 2018;30(1):9-17. [PMID: 28791876]
Gibas K. M., van den Berg P., Powell V. E., et al. Drug resistance during HIV pre-exposure prophylaxis. Drugs 2019;79(6):609-19. [PMID: 30963509]
Girometti N., McCormack S., Tittle V., et al. Rising rates of recent PrEP exposure among MSM newly diagnosed with HIV: antiviral resistance patterns and treatment outcomes. AIDS 2022;36(4):561-66. [PMID: 34873084]
Glidden D. V., Amico K. R., Liu A. Y., et al. Symptoms, side effects and adherence in the iPrEx open-label extension. Clin Infect Dis 2016;62(9):1172-77. [PMID: 26797207]
Goedel W. C., King M. R., Lurie M. N., et al. Effect of racial inequities in pre-exposure prophylaxis use on racial disparities in HIV incidence among men who have sex with men: a modeling study. J Acquir Immune Defic Syndr 2018;79(3):323-29. [PMID: 30044303]
Goldstein R. H., Streed C. G., Cahill S. R. Being PrEPared - preexposure prophylaxis and HIV disparities. N Engl J Med 2018;379(14):1293-95. [PMID: 30230965]
Golub S. A. Tensions between the epidemiology and psychology of HIV risk: implications for pre-exposure prophylaxis. AIDS Behav 2014;18(9):1686-93. [PMID: 24719201]
Golub S. A., Pena S., Boonrai K., et al. STI data from community-based PrEP implementation suggest changes to CDC guidelines. CROI; 2016 Feb 22-25. http://www.croiconference.org/sessions/sti-data-community-based-prep-implementation-suggest-changes-cdc-guidelines-0
Goodreau S. M., Hamilton D. T., Jenness S. M., et al. Targeting human immunodeficiency virus pre-exposure prophylaxis to adolescent sexual minority males in higher prevalence areas of the United States: a modeling study. J Adolesc Health 2018;62(3):311-19. [PMID: 29248392]
Grant R. M., Anderson P. L., McMahan V., et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014;14(9):820-29. [PMID: 25065857]
Grant R. M., Lama J. R., Anderson P. L., et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363(27):2587-99. [PMID: 21091279]
Grant R. M., Pellegrini M., Defechereux P. A., et al. Sex hormone therapy and tenofovir diphosphate concentration in dried blood spots: primary results of the interactions between antiretrovirals and transgender hormones study. Clin Infect Dis 2021;73(7):e2117-23. [PMID: 32766890]
Grov C., Rendina H. J., Ventuneac A., et al. HIV risk in group sexual encounters: an event-level analysis from a national online survey of MSM in the U.S. J Sex Med 2013;10(9):2285-94. [PMID: 23809410]
Hamilton D. T., Rosenberg E. S., Jenness S. M., et al. Modeling the joint effects of adolescent and adult PrEP for sexual minority males in the United States. PLoS One 2019;14(5):e0217315. [PMID: 31116802]
Havens P. L., Perumean-Chaney S. E., Patki A., et al. Changes in bone mass after discontinuation of pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network Protocols 110 and 113. Clin Infect Dis 2020;70(4):687-91. [PMID: 31179503]
Havens P. L., Stephensen C. B., Van Loan M. D., et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis 2017;64(3):317-25. [PMID: 28013265]
Heffron R., Pintye J., Matthews L. T., et al. PrEP as peri-conception HIV prevention for women and men. Curr HIV/AIDS Rep 2016;13(3):131-39. [PMID: 26993627]
Hendrix C. W., Andrade A., Bumpus N. N., et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016;32(1):32-43. [PMID: 26414912]
Heuker J., Sonder G. J., Stolte I., et al. High HIV incidence among MSM prescribed postexposure prophylaxis, 2000-2009: indications for ongoing sexual risk behaviour. Aids 2012;26(4):505-12. [PMID: 22156963]
Hill A., Hughes S. L., Gotham D., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety?. J Virus Erad 2018;4(2):72-79. [PMID: 29682298]
Hiransuthikul A., Janamnuaysook R., Himmad K., et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J Int AIDS Soc 2019;22(7):e25338. [PMID: 31298497]
Hojilla C. J., Koester K. A., Cohen S. E., et al. Sexual behavior, risk compensation, and HIV prevention strategies among participants in the San Francisco PrEP Demonstration Project: a qualitative analysis of counseling notes. AIDS Behav 2016;20(7):1461-69. [PMID: 25835463]
Hoornenborg(a) E., Prins M., Achterbergh R. C. A., et al. Acquisition of wild-type HIV-1 infection in a patient on pre-exposure prophylaxis with high intracellular concentrations of tenofovir diphosphate: a case report. Lancet HIV 2017;4(11):e522-28. [PMID: 28919303]
Hoornenborg(b) E., Achterbergh R. C. A., Schim Van Der Loeff M. F., et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS 2017;31(11):1603-10. [PMID: 28657964]
Hosek S. G., Rudy B., Landovitz R., et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr 2017;74(1):21-29. [PMID: 27632233]
Jarlais D. D. Systematic review research on needle/syringe programs and opiate substitution programs in low- and middle-income countries. J Food Drug Anal 2013;21(4):s59-61. [PMID: 25267887]
Jenness S. M., Goodreau S. M., Rosenberg E., et al. Impact of the Centers for Disease Control's HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis 2016;214(12):1800-1807. [PMID: 27418048]
Jenness S. M., Maloney K. M., Smith D. K., et al. Addressing gaps in HIV preexposure prophylaxis care to reduce racial disparities in HIV incidence in the United States. Am J Epidemiol 2019;188(4):743-52. [PMID: 30312365]
Jenness S. M., Weiss K. M., Goodreau S. M., et al. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis 2017;65(5):712-18. [PMID: 28505240]
Johnson M. O., Sevelius J. M., Dilworth S. E., et al. Preliminary support for the construct of health care empowerment in the context of treatment for human immunodeficiency virus. Patient Prefer Adherence 2012;6:395-404. [PMID: 22654510]
Kamis K. F., Marx G. E., Scott K. A., et al. Same-day HIV pre-exposure prophylaxis (PrEP) initiation during drop-in sexually transmitted diseases clinic appointments is a highly acceptable, feasible, and safe model that engages individuals at risk for HIV into PrEP care. Open Forum Infect Dis 2019;6(7):ofz310. [PMID: 31341933]
King H. L., Keller S. B., Giancola M. A., et al. Pre-exposure prophylaxis accessibility research and evaluation (PrEPARE Study). AIDS Behav 2014;18(9):1722-25. [PMID: 25017425]
Knox D. C., Anderson P. L., Harrigan P. R., et al. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. N Engl J Med 2017;376(5):501-2. [PMID: 28146652]
Koblin B. A., Mansergh G., Frye V., et al. Condom-use decision making in the context of hypothetical pre-exposure prophylaxis efficacy among substance-using men who have sex with men: Project MIX. J Acquir Immune Defic Syndr 2011;58(3):319-27. [PMID: 21765363]
Koren D. E., Fedkiv V., Zhao H., et al. Perceptions of long-acting injectable antiretroviral treatment regimens in a United States urban academic medical center. J Int Assoc Provid AIDS Care 2020;19:2325958220981265. [PMID: 33327851]
Kuhns L. M., Reisner S. L., Mimiaga M. J., et al. Correlates of PrEP indication in a multi-site cohort of young HIV-uninfected transgender women. AIDS Behav 2016;20(7):1470-77. [PMID: 26336946]
LaLota M., Beck D. W., Metsch L. R., et al. HIV seropositivity and correlates of infection among heterosexually active adults in high-risk areas in South Florida. AIDS Behav 2011;15(6):1259-63. [PMID: 21153433]
Lampe M. A., Smith D. K., Anderson G. J., et al. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am J Obstet Gynecol 2011;204(6):488.e1-8. [PMID: 21457911]
Lancki N., Almirol E., Alon L., et al. Preexposure prophylaxis guidelines have low sensitivity for identifying seroconverters in a sample of young Black MSM in Chicago. AIDS 2018;32(3):383-92. [PMID: 29194116]
Landovitz R. J., Donnell D., Clement M. E., et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021;385(7):595-608. [PMID: 34379922]
Landovitz R. J., Donnell D., Tran H., et al. Updated efficacy, safety, and case studies in HPTN 083: CAB-LA vs TDF/FTC for PrEP. CROI; 2022 Feb 12-16. https://www.croiconference.org/abstract/updated-efficacy-safety-and-case-studies-in-hptn-083-cab-la-vs-tdf-ftc-for-prep/
Landovitz R. J., Li S., Eron J. J., et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 2020;7(7):e472-81. [PMID: 32497491]
Lehman D. A., Baeten J. M., McCoy C. O., et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis 2015;211(8):1211-18. [PMID: 25587020]
Liotta G., Floridia M., Andreotti M., et al. Growth indices in breastfed infants pre and postnatally exposed to tenofovir compared with tenofovir-unexposed infants. Aids 2016;30(3):525-27. [PMID: 26765942]
Liu A. Y., Cohen S. E., Vittinghoff E., et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016;176(1):75-84. [PMID: 26571482]
Liu A. Y., Hessol N. A., Vittinghoff E., et al. Medication adherence among men who have sex with men at risk for HIV infection in the United States: implications for pre-exposure prophylaxis implementation. AIDS Patient Care STDS 2014;28(12):622-27. [PMID: 25396706]
Liu A. Y., Vittinghoff E., Chillag K., et al. Sexual risk behavior among HIV-uninfected men who have sex with men participating in a tenofovir preexposure prophylaxis (PrEP) randomized trial in the United States. J Acquir Immune Defic Syndr 2013;64(1):87-94. [PMID: 23481668]
Louissaint N. A., Cao Y. J., Skipper P. L., et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013;29(11):1443-50. [PMID: 23600365]
Lunny C., Taylor D., Hoang L., et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015;10(7):e0132776. [PMID: 26168051]
Marcus J. L., Buisker T., Horvath T., et al. Helping our patients take HIV pre-exposure prophylaxis (PrEP): a systematic review of adherence interventions. HIV Med 2014;15(7):385-95. [PMID: 24580813]
Markowitz M., Grossman H., Anderson P. L., et al. Newly acquired infection with multidrug-resistant HIV-1 in a patient adherent to preexposure prophylaxis. J Acquir Immune Defic Syndr 2017;76(4):e104-6. [PMID: 29076941]
Marrazzo J. M., del Rio C., Holtgrave D. R., et al. HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 2014;312(4):390-409. [PMID: 25038358]
Marzinke(a) M., Grinsztejn B., Fogel J. M., et al. Characterization of human immunodeficiency virus (HIV) infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J Infect Dis 2021;224(9):1581-92. [PMID: 33740057]
Marzinke(b) M., Delany-Moretlwe S., Agyei Y., et al. Long-acting injectable PrEP in women: laboratory analysis of HIV infections in HPTN 084. IAS; 2021 Jul 18-21. https://theprogramme.ias2021.org/Abstract/Abstract/2606
Massud I., Cong M. E., Ruone S., et al. Efficacy of oral tenofovir alafenamide/emtricitabine combination or single-agent tenofovir alafenamide against vaginal simian human immunodeficiency virus infection in macaques. J Infect Dis 2019;220(11):1826-33. [PMID: 31362305]
Mayer K. H., Grasso C., Levine K., et al. Increasing PrEP uptake, persistent disparities in at-risk patients in a Boston center. CROI; 2018 Mar 4-7. http://www.croiconference.org/sessions/increasing-prep-uptake-persistent-disparities-risk-patients-boston-center
Mayer K. H., Molina J. M., Thompson M. A., et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020;396(10246):239-54. [PMID: 32711800]
McCormack S., Dunn D. T., Desai M., et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387(10013):53-60. [PMID: 26364263]
McFaul K., Maghlaoui A., Nzuruba M., et al. Acute hepatitis C infection in HIV-negative men who have sex with men. J Viral Hepat 2015;22(6):535-38. [PMID: 25412826]
Mehrotra M. L., Westreich D., McMahan V. M., et al. Baseline characteristics explain differences in effectiveness of randomization to daily oral TDF/FTC PrEP between transgender women and cisgender men who have sex with men in the iPrEx trial. J Acquir Immune Defic Syndr 2019;81(3):e94-98. [PMID: 31192894]
Meyers K., Rodriguez K., Moeller R. W., et al. High interest in a long-acting injectable formulation of pre-exposure prophylaxis for HIV in young men who have sex with men in NYC: a P18 cohort substudy. PLoS One 2014;9(12):e114700. [PMID: 25502768]
Mikati T., Jamison K., Daskalakis D. C. Immediate PrEP initiation at New York City sexual health clinics. CROI; 2019 Mar 4-7. http://www.croiconference.org/sessions/immediate-prep-initiation-new-york-city-sexual-health-clinics
Molina J. M., Capitant C., Spire B., et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015;373(23):2237-46. [PMID: 26624850]
Molina J. M., Ghosn J., Assoumou L., et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV 2022;9(8):e554-62. [PMID: 35772417]
Morgan E., Ryan D. T., Newcomb M. E., et al. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav 2018;22(11):3645-48. [PMID: 29728950]
Mugo N. R., Hong T., Celum C., et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA 2014;312(4):362-71. [PMID: 25038355]
Mugwanya K. K., Hendrix C. W., Mugo N. R., et al. Pre-exposure prophylaxis use by breastfeeding HIV-uninfected women: a prospective short-term study of antiretroviral excretion in breast milk and infant absorption. PLoS Med 2016;13(9):e1002132. [PMID: 27676257]
Mujugira A., Celum C., Coombs R. W., et al. HIV transmission risk persists during the first 6 months of antiretroviral therapy. J Acquir Immune Defic Syndr 2016;72(5):579-84. [PMID: 27070123]
Murray M. I., Markowitz M., Frank I., et al. Satisfaction and acceptability of cabotegravir long-acting injectable suspension for prevention of HIV: patient perspectives from the ECLAIR trial. HIV Clin Trials 2018;19(4):129-138. [PMID: 30445896]
NYC Health. 2016 DOHMH Health Alert #49: Potential breakthrough HIV infection in patient on pre-exposure prophylaxis. 2016 Oct 27. https://www1.nyc.gov/assets/doh/downloads/pdf/hcp/han-PrEP-breakthrough.pdf [accessed 2022 Mar 14]
NYSDOH. Dear colleague letter. 2017 Sep. https://www.health.ny.gov/diseases/aids/ending_the_epidemic/docs/september_physician_letter.pdf [accessed 2022 Mar 17]
NYSDOH. Unpublished data. 2018.
Ogbuagu O., Ruane P. J., Podzamczer D., et al. Long-term safety and efficacy of emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV-1 pre-exposure prophylaxis: week 96 results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet HIV 2021;8(7):e397-407. [PMID: 34197772]
Orkin C., Bernal Morell E., Tan D. H. S., et al. Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study. Lancet HIV 2021;8(11):e668-78. [PMID: 34656207]
Orkin C., Molina J. M., Negredo E., et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV 2018;5(1):e23-34. [PMID: 28993180]
Page K. R., Martinez O., Nieves-Lugo K., et al. Promoting pre-exposure prophylaxis to prevent HIV infections among sexual and gender minority Hispanics/Latinxs. AIDS Educ Prev 2017;29(5):389-400. [PMID: 29068715]
Patterson K. B., Prince H. A., Kraft E., et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011;3(112):112re4. [PMID: 22158861]
Patton M. E., Kidd S., Llata E., et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men--STD Surveillance Network, United States, 2010–2012. Clin Infect Dis 2014;58(11):1564-70. [PMID: 24647015]
Philbin M. M., Parish C., Bergen S., et al. A qualitative exploration of women's interest in long-acting injectable antiretroviral therapy across six cities in the women's interagency HIV study: intersections with current and past injectable medication and substance use. AIDS Patient Care STDS 2021;35(1):23-30. [PMID: 33400587]
Philbin M. M., Parker C. M., Parker R. G., et al. The promise of pre-exposure prophylaxis for black men who have sex with men: an ecological approach to attitudes, beliefs, and barriers. AIDS Patient Care STDS 2016;30(6):282-90. [PMID: 27220036]
Pilkington V., Hill A., Hughes S., et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad 2018;4(4):215-24. [PMID: 30515300]
Pitasi M. A., Kerani R. P., Kohn R., et al. Chlamydia, gonorrhea, and human immunodeficiency virus infection among transgender women and transgender men attending clinics that provide sexually transmitted disease services in six US cities: results from the Sexually Transmitted Disease Surveillance Network. Sex Transm Dis 2019;46(2):112-17. [PMID: 30278030]
Prevention Access Campaign. Risk of sexual transmission of HIV from a person living with HIV who has an undetectable viral load. Messaging primer & consensus statement. 2019 Jan 28. https://preventionaccess.org/ [accessed 2022 Mar 14]
Price J. C., McKinney J. E., Crouch P. C., et al. Sexually acquired hepatitis C infection in HIV-uninfected men who have sex with men using pre-exposure prophylaxis against HIV. J Infect Dis 2019;219(9):1373-76. [PMID: 30462305]
Rael C. T., Martinez M., Giguere R., et al. Barriers and facilitators to oral PrEP use among transgender women in New York City. AIDS Behav 2018;22(11):3627-36. [PMID: 29589137]
Rael C. T., Martinez M., Giguere R., et al. Transgender women's concerns and preferences on potential future long-acting biomedical HIV prevention strategies: the case of injections and implanted medication delivery devices (IMDDs). AIDS Behav 2020;24(5):1452-62. [PMID: 31654172]
Reed J. B., Shrestha P., Were D., et al. HIV PrEP is more than ART-lite: longitudinal study of real-world PrEP services data identifies missing measures meaningful to HIV prevention programming. J Int AIDS Soc 2021;24(10):e25827. [PMID: 34648678]
Rizzardini G., Overton E. T., Orkin C., et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: week 48 pooled analysis of phase 3 ATLAS and FLAIR trials. J Acquir Immune Defic Syndr 2020;85(4):498-506. [PMID: 33136751]
Rodger A. J., Cambiano V., Bruun T., et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. Jama 2016;316(2):171-81. [PMID: 27404185]
Salvadori N., Fan B., Teeyasoontranon W., et al. Maternal and infant bone mineral density 1 year after delivery in a randomized, controlled trial of maternal tenofovir disoproxil fumarate to prevent mother-to-child transmission of hepatitis B virus. Clin Infect Dis 2019;69(1):144-46. [PMID: 30924492]
Scheim A. I., Bauer G. R., Travers R. HIV-related sexual risk among transgender men who are gay, bisexual, or have sex with men. J Acquir Immune Defic Syndr 2017;74(4):e89-96. [PMID: 27798432]
Scott H. M., Spinelli M., Vittinghoff E., et al. Racial/ethnic and HIV risk category disparities in preexposure prophylaxis discontinuation among patients in publicly funded primary care clinics. AIDS 2019;33(14):2189-95. [PMID: 31436610]
Seifert S. M., Chen X., Meditz A. L., et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses 2016;32(10-11):981-91. [PMID: 27526873]
Serota D. P., Rosenberg E. S., Sullivan P. S., et al. Pre-exposure prophylaxis uptake and discontinuation among young black men who have sex with men in Atlanta, Georgia: a prospective cohort study. Clin Infect Dis 2020;71(3):574-82. [PMID: 31499518]
Sevelius J. M., Deutsch M. B., Grant R. The future of PrEP among transgender women: the critical role of gender affirmation in research and clinical practices. J Int AIDS Soc 2016;19(7 Suppl 6):21105. [PMID: 27760683]
Shieh E., Marzinke M. A., Fuchs E. J., et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J Int AIDS Soc 2019;22(11):e25405. [PMID: 31692269]
Siberry G. K., Jacobson D. L., Kalkwarf H. J., et al. Lower newborn bone mineral content associated with maternal use of tenofovir disoproxil fumarate during pregnancy. Clin Infect Dis 2015;61(6):996-1003. [PMID: 26060285]
Sidebottom D., Ekstrom A. M., Stromdahl S. A systematic review of adherence to oral pre-exposure prophylaxis for HIV - how can we improve uptake and adherence?. BMC Infect Dis 2018;18(1):581. [PMID: 30445925]
Siegler A. J., Mayer K. H., Liu A. Y., et al. Developing and assessing the feasibility of a home-based PrEP monitoring and support program. Clin Infect Dis 2019;63(3):501-4. [PMID: 29982304]
Singh S., Lampe M. A., Surendera A. B., et al. HIV seroconversion during pregnancy and mother-to-child HIV transmission: data from the Enhanced Perinatal Surveillance Project, United States, 2005-2010. THAC0104. JIAS 2012;15:130-31. https://studylib.net/doc/18607036/--journal-of-the-international-aids-society
Smith D. K., Pals S. L., Herbst J. H., et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2012;60(4):421-27. [PMID: 22487585]
Smith D. K., Switzer W. M., Peters P., et al. A strategy for PrEP clinicians to manage ambiguous HIV test results during follow-up visits. Open Forum Infect Dis 2018;5(8):ofy180. [PMID: 30568989]
Spinelli M. A., Glidden D. V., Anderson P. L., et al. Impact of estimated pre-exposure prophylaxis (PrEP) adherence patterns on bone mineral density in a large PrEP demonstration project. AIDS Res Hum Retroviruses 2019;35(9):788-93. [PMID: 31119944]
Sullivan P. S., Giler R. M., Mouhanna F., et al. Trends in the use of oral emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against HIV infection, United States, 2012-2017. Ann Epidemiol 2018;28(12):833-840. [PMID: 30037634]
Tang E. C., Vittinghoff E., Philip S. S., et al. Quarterly screening optimizes detection of sexually transmitted infections when prescribing HIV preexposure prophylaxis. AIDS 2020;34(8):1181-86. [PMID: 32205724]
Taylor D., Lunny C., Wong T., et al. Self-collected versus clinician-collected sampling for sexually transmitted infections: a systematic review and meta-analysis protocol. Syst Rev 2013;2(1):93. [PMID: 24112441]
Thigpen M. C., Kebaabetswe P. M., Paxton L. A., et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367(5):423-34. [PMID: 22784038]
Thomson K. A., Hughes J., Baeten J. M., et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis 2018;218(1):16-25. [PMID: 29514254]
Tolley E. E., Zangeneh S. Z., Chau G., et al. Acceptability of long-acting injectable cabotegravir (CAB LA) in HIV-uninfected individuals: HPTN 077. AIDS Behav 2020;24(9):2520-31. [PMID: 32052214]
Townes A.R., Tanner M., Henry K.D., et al. Low proportions of linkage & prescriptions of PrEP in Black women (THRIVE, 2015-2020). CROI; 2021 Mar 6-10. https://www.croiconference.org/abstract/low-proportions-of-linkage-prescriptions-of-prep-in-black-women-thrive-2015-2020/
Traeger M. W., Schroeder S. E., Wright E. J., et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018;67(5):676-86. [PMID: 29509889]
USPSTF. Preexposure prophylaxis to prevent acquisition of HIV: US Preventive Services Task Force recommendation statement. JAMA 2023;330(8):736-45. [PMID: 37606666]
Van Damme L., Corneli A., Ahmed K., et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012;367(5):411-22. [PMID: 22784040]
van de Vijver D. A., Mukherjee S., van Kampen J.J. Antiretroviral drug treatment of individuals that used preexposure prophylaxis (PrEP) before diagnosis. Curr Treat Options Infect Dis 2021;13:141-52. https://doi.org/10.1007/s40506-021-00246-9
van der Sluis W. B., Bouman M. B., Gijs L., et al. Gonorrhoea of the sigmoid neovagina in a male-to-female transgender. Int J STD AIDS 2015;26(8):595-98. [PMID: 25060698]
Vanhommerig J. W., Lambers F. A., Schinkel J., et al. Risk factors for sexual transmission of hepatitis C virus among human immunodeficiency virus-infected men who have sex with men: a case-control study. Open Forum Infect Dis 2015;2(3):ofv115. [PMID: 26634219]
Velloza J., Bacchetti P., Hendrix C. W., et al. Short- and long-term pharmacologic measures of HIV pre-exposure prophylaxis use among high-risk men who have sex with men in HPTN 067/ADAPT. J Acquir Immune Defic Syndr 2019;82(2):149-58. [PMID: 31335588]
Vernazza P. L., Graf I., Sonnenberg-Schwan U., et al. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011;25(16):2005-8. [PMID: 21716070]
Vigano A., Mora S., Giacomet V., et al. In utero exposure to tenofovir disoproxil fumarate does not impair growth and bone health in HIV-uninfected children born to HIV-infected mothers. Antivir Ther 2011;16(8):1259-66. [PMID: 22155907]
Wagner G. A., Wu K. S., Anderson C., et al. Predictors of human immunodeficiency virus pre-exposure prophylaxis (PrEP) uptake in a sexual health clinic with rapid PrEP initiation. Open Forum Infect Dis 2023;10(3):ofad060. [PMID: 36968957]
Were E. O., Heffron R., Mugo N. R., et al. Pre-exposure prophylaxis does not affect the fertility of HIV-1-uninfected men. AIDS 2014;28(13):1977-82. [PMID: 25259704]
Werner R. N., Gaskins M., Nast A., et al. Incidence of sexually transmitted infections in men who have sex with men and who are at substantial risk of HIV infection - a meta-analysis of data from trials and observational studies of HIV pre-exposure prophylaxis. PLoS One 2018;13(12):e0208107. [PMID: 30507962]
WHO. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection. 2017 Jul. https://apps.who.int/iris/bitstream/handle/10665/255889/WHO-HIV-2017.17-eng.pdf;jsessionid=18A1D4D7DFF045A5B4A2EA2F8D6BCFDE?sequence=1 [accessed 2022 Mar 17]
Workowski K. A., Bolan G. A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64(RR-03):1-137. [PMID: 26042815]
Zetola N. M., Bernstein K. T., Wong E., et al. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. J Acquir Immune Defic Syndr 2009;50(5):546-51. [PMID: 19367993]
Zucker J., Carnevale C., Rai A. J., et al. Positive or not, that is the question: HIV testing for individuals on pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2018;78(2):e11-13. [PMID: 29481487]
Zule W. A., Costenbader E. C., Meyer W. J., et al. Methamphetamine use and risky sexual behaviors during heterosexual encounters. Sex Transm Dis 2007;34(9):689-94. [PMID: 17471112]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | October 10, 2019 |
| Date of current publication | May 20, 2022 |
| Highlights of changes, additions, and updates in the May 20, 2022 edition |
Updates were made to incorporate data that are new since the previous edition was published in Aug. 2021 and to include recommendations for the long-acting injectable formulation of the integrase strand transfer inhibitor cabotegravir as PrEP |
| Intended users | New York State clinicians who are recommending, initiating, or maintaining PrEP care for individuals at risk of acquiring HIV |
| Lead author |
Rona M. Vail, MD |
| Writing group |
Steven M. Fine, MD, PhD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Jessica Rodrigues, ; Christopher J. Hoffmann, MD, MPH; Lyn C. Stevens, MS, NP, ACRN; Charles J. Gonzalez, MD |
| Author and writing group conflict of interest disclosures |
Joseph P. McGowan, MD, FACP, FIDSA: Institutional Pharma grant recipient/support, clinical trial; Gilead |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines |
NYSDOH AI Guidance |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on January 30, 2024

