Purpose of This Guideline
Date of current publication: April 17, 2023
Lead author: Elliott DeHaan, MD
Contributors: Christine A. Kerr, MD; Aracelis Fernandez, MD; Lisa-Gaye Robinson, MD; Ruby Fayorsey, MD
Writing group: Steven M. Fine, MD, PhD; Rona Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: June 25, 2020
| NEW IN THE 2020 EDITION OF THIS GUIDELINE |
|
Elliot DeHaan, MD, lead author of this guideline, discusses what’s new in an interview with TheBodyPro (8/11/20). Reorganization of the previous 4 guidelines into 1 document: This PEP guideline addresses management of 4 types of exposure to HIV: occupational, non-occupational (consensual sexual exposure, exposure through needle-sharing), sexual assault, and exposures in children. Icons throughout signal content specific to one exposure type (see the icon key below). This edition reflects a unified approach to the recommendations for all exposure types, with differences between exposure scenarios highlighted throughout. With updated recommendations for:
Additional highlights:
|
This guideline was developed by the New York State Department of Health (NYSDOH) AIDS Institute (AI) for healthcare practitioners in any medical setting (e.g., emergency department, sexual health clinic, urgent care clinic, inpatient unit primary care practice) who manage the care of individuals who request post-exposure prophylaxis (PEP) after a possible exposure to HIV. Despite the availability of prevention measures, exposures occur that pose the risk of transmission. Fortunately, with rapid initiation of PEP, infection can be blocked. Preventing new HIV infections is crucial to the success of New York State’s Ending the Epidemic Initiative.
HIV transmission can be prevented through use of barrier protection during sex (e.g., latex condoms), safer drug injection techniques, and adherence to universal precautions in the healthcare setting. HIV infection can also be prevented with use of antiretroviral (ARV) medications taken as pre-exposure prophylaxis (PrEP). After an exposure has occurred, HIV infection can be prevented with rapid administration of ARV medications as PEP. The first dose of PEP should be administered within 2 hours of an exposure (ideal) and no later than 72 hours after an exposure.
| KEY POINTS |
|
In addition to clinical recommendations, this guideline details selected good practices and highlights laws and legal considerations that are pertinent in delivering PEP care.
Goals: This guideline aims to achieve the following goals:
- Prevent HIV infection in individuals who experience a high-risk exposure.
- Reinforce that HIV exposure is an emergency that requires rapid response, with immediate administration of the first dose of PEP medications.
- Reduce under- and over-prescribing of PEP by describing the benefits of PEP and providing guidance for identifying high-risk HIV exposures for which PEP is indicated.
- Ensure prescription of PEP regimens that are effective and well tolerated.
- Assist clinicians in recognizing and addressing challenges to successful completion of a PEP regimen.
- Detail the baseline testing, monitoring, and follow-up that should accompany prescription of a 28-day course of PEP.
- Assist clinicians in managing potential concurrent exposures to hepatitis B virus (HBV) and hepatitis C virus (HCV).
Note on “experienced” and “expert” HIV care providers: Throughout this guideline, when reference is made to “experienced HIV care provider” or “expert HIV care provider,” those terms are referring to the following 2017 NYSDOH AI definitions:
- Experienced HIV care provider: Practitioners who have been accorded HIV Experienced Provider status by the American Academy of HIV Medicine or have met the HIV Medicine Association’s definition of an experienced provider are eligible for designation as an HIV Experienced Provider in New York State. Nurse practitioners and licensed midwives who provide clinical care to individuals with HIV in collaboration with a physician may be considered HIV Experienced Providers as long as all other practice agreements are met (8 NYCRR 79-5:1; 10 NYCRR 85.36; 8 NYCRR 139-6900). Physician assistants who provide clinical care to individuals with HIV under the supervision of an HIV Specialist physician may also be considered HIV Experienced Providers (10 NYCRR 94.2)
- Expert HIV care provider: A provider with extensive experience in the management of complex patients with HIV.
| Icon Key |
All exposures Occupational exposures Non-occupational exposures Sexual assault exposures Exposures in children aged 2 to 12 years |
Risk of Infection Following an Exposure to HIV
Factors that increase the risk of transmission: Many factors that contribute to HIV infection are shared by the 4 PEP scenarios outlined below. HIV transmission risk depends on the viral load of the source with HIV and the type of exposure Sultan, et al. 2014. Factors that increase the risk of HIV transmission include early- and late-stage untreated HIV infection and a high level of HIV RNA in the blood Cardo, et al. 1997, the presence of genital or anorectal ulcers from sexually transmitted infections (STIs), and direct blood-to-blood exchange, such as syringe sharing during injection drug use Kaplan and Heimer 1992; PRN Notebook 2005; Johnson and Lewis 2008; Mayer and Venkatesh 2011; Wall, et al. 2017.
Factors that decrease the risk of HIV transmission: Similarly, across the 4 PEP scenarios, there are shared factors that decrease the risk of HIV infection. HIV transmission risk is low and often negligible when the source of the exposure has a low or undetectable viral load Rodger, et al. 2016; Rodger, et al. 2019 and is lower if the source is circumcised (if a cis-gender male and the circumcision is healed) Auvert, et al. 2005; Bailey, et al. 2007; Gray, et al. 2007 or is taking antiretroviral medications as pre-exposure prophylaxis (PrEP) Grant, et al. 2010; Baeten, et al. 2012. In the context of sexual exposure, there is a robust body of evidence that individuals do not sexually transmit HIV if they are taking antiretroviral therapy (ART) and have an undetectable viral load (HIV RNA <200 copies/mL). Data are insufficient to make recommendations regarding HIV transmission via breastfeeding.
Occupational Exposure Risk
The risk of HIV transmission in a healthcare setting has been reported as 0.3% through percutaneous exposure to the blood of a source with HIV Cardo, et al. 1997 and 0.09% after a mucous membrane exposure Kuhar, et al. 2013. In the Centers for Disease Control and Prevention (CDC) Needlestick Surveillance Group study, use of zidovudine (as post-exposure prophylaxis [PEP]) by healthcare workers reduced the risk of HIV acquisition by 81% overall for percutaneous exposures Cardo, et al. 1997. With the use of potent antiretroviral (ARV) medications that have increased bioavailability, it is presumed the use of a 3-drug PEP regimen would significantly reduce this risk further.
In the current era of increasing viral suppression in patients with HIV, early and appropriate PEP initiation, and improved infection control protocols, these rates may be lower. In one cohort of 266 healthcare workers who had percutaneous or mucocutaneous injuries and exposure to HIV-contaminated body fluids, there were zero seroconversions over a 13-year period (seroconversion rate 0%). In addition to their internal findings, the authors compared their results to a calculated overall HIV seroconversion rate of 0.13% after a literature review conducted in October 2016 yielded 17 articles that documented 10 seroconversions among 7,652 healthcare-related exposures Nwaiwu, et al. 2017.
The mean risk may be significantly higher in cases of percutaneous exposure in which more than 1 risk factor is present (e.g., in individuals who incur a deep injury with a hollow-bore needle from a source with HIV and a high viral load). Although the effect of viral load level has not been studied in the patients with occupational exposures, there is evidence that the probability of sexually transmitting HIV is correlated with the source’s HIV viral load Quinn, et al. 2000; Modjarrad, et al. 2008; Attia, et al. 2009.
Prevention of occupational exposure: As part of the employer’s plan to prevent transmission of bloodborne pathogens, the following measures can be taken to avoid injuries:
- Eliminate unnecessary use of needles and other sharps.
- Ensure use of and compliance with devices with safety features.
- Eliminate needle recapping.
- Ensure safe handling and prompt disposal of needles in containers for sharps disposal.
- Provide ongoing education about and promote safe work practices for handling needles and other sharps.
For more information about prevention of needlestick injuries, refer to the NIOSH Alert: Preventing Needlestick Injuries in Health Care Settings NIOSH 1999.
| Resources |
|
Even when effective prevention measures are implemented, exposures to blood and bodily fluid still occur. Employers of personnel covered by the OSHA Bloodborne Pathogen Standard are obligated to provide post-exposure care, including prophylaxis, at no cost to the employee. The employer may subsequently attempt to obtain reimbursement from Workers’ Compensation. For more information, see Employer Responsibilities in Management of PEP to Prevent HIV Infection Following an Occupational Exposure.
Non-Occupational Exposure Risk
| Box 1: Risk per 10,000 Exposures of Acquiring HIV From an Infected Source and Factors That Increase Risk Modified from the Centers for Disease Control and Prevention CDC(a) 2019. |
Parenteral Exposure Risk:
Factors that increase risk of transmission through parenteral exposure:
Sexual Exposure Risk:
Factors that increase risk of transmission through sexual exposure:
Other Exposure Types:
Factors that increase risk of transmission through other exposures:
|
Sexual exposures (consensual): Exposures that may prompt a request for non-occupational PEP include condom slippage or breakage; lapse in condom use by serodiscordant or unknown status partners; or other episodic exposure to blood or other potentially infectious body fluids, including semen, vaginal secretions, or body fluids with visible blood contamination. In addition to the viral load of a source with HIV, other factors that influence transmission and acquisition risk include Sultan, et al. 2014:
- Genitorectal trauma
- Type of sexual exposure, i.e., receptive anal, receptive vaginal, insertive anal, insertive vaginal, receptive oral
- Presence of STIs and genital/anal ulcers
- Circumcision status
Condomless receptive anal sex with and without ejaculation carries a risk of 1.43% and 0.65%, respectively. Condomless insertive anal intercourse carries a risk of 0.62% in uncircumcised men and 0.11% in circumcised men Jin, et al. 2010. In one European study, the risk associated with condomless receptive and insertive vaginal intercourse was 0.08% and 0.04%, respectively Mastro and de Vincenzi 1996. Information for patients is available about correct male (insertive) and female (receptive) condom use.
The CDC’s HIV Risk Reduction Tool can help identify an individual’s risk of acquiring HIV.
Needle sharing and needlestick injuries: Needle sharing among injection drug users is a common reason to request PEP, as the associated risk has been estimated to be as high as 63 per 10,000 exposures based on a study among injection drug users in Thailand Hudgens, et al. 2001; Hudgens, et al. 2002. For this reason, PEP should always be considered in this scenario provided the potential exposure was within 72 hours.
Another route of exposure that prompts requests for PEP is needlestick injury in the community. Factors associated with risk from needlestick injuries include the potential source of the needle, type of needle, presence of blood, and skin penetration.
Individuals who incur needlestick injuries from discarded needles are often concerned about potential HIV exposure. Consideration of potential risk from discarded needles should include the prevalence of HIV in the community or facility where the exposure occurred and the prevalence of injection drug use in the surrounding area. However, the risk of HIV transmission through exposure to dried blood found on syringes is extremely low Zamora, et al. 1998. Discarded needles should not be tested for HIV because of low yield and the risk of injury to personnel involved in the testing.
Vaccination to prevent tetanus and administration of hepatitis B vaccine are indicated for needlestick injures resulting in puncture wounds, based on immunization history and hepatitis B virus status of the source Medscape 2021; Bader and McKinsey 2013. Hepatitis B immunoglobulin may also be necessary (see guideline sections Management of Potential Exposure to Hepatitis B Virus and Management of Potential Exposure to Hepatitis C Virus).
Bite wounds: An estimated 250,000 human bites occur annually in the United States in a variety of settings American Academy of Pediatrics 1997. Although possible, HIV transmission through bites is thought to be extremely rare. Though many reported instances of bites have occurred, few cases of associated HIV infection have been established. Cases of possible HIV transmission have been documented following bites in adults exposed to blood-tinged saliva Vidmar, et al. 1996; Pretty, et al. 1999. A systematic review found no cases of HIV transmission through spitting and 9 possible cases of HIV transmission through a bite (6 occurred between family members, and 2 involved untrained first responders who placed their fingers in the mouth of an individual who is experiencing a seizure). Only 4 of the 9 cases were confirmed or classified as highly plausible Cresswell, et al. 2018.
A bite wound that results in blood exposure should prompt consideration of PEP. When a human bite occurs, it is possible for both the individual who was bitten and the biter to incur blood exposure (see scenarios listed below). Use of PEP in such a case may be indicated if there is significant exposure to deep, bloody wounds. A bite is not considered a risk exposure to either party when the integrity of the skin is not disrupted.
Scenarios in which bites may result in blood exposure:
- Blood exposure to the biter: When the biter inflicts a wound that breaks the skin and blood from the bitten individual enters the biter’s mouth.
- Blood exposure to the bitten individual: When the biter has blood in his or her mouth (e.g., from bleeding gums or lesions) and inflicts a wound that breaks the skin of the individual bitten.
- Blood exposure to both parties: A break in the skin of the individual who was bitten and the biter has blood in his/her mouth (e.g., from bleeding gums or lesions).
Prevention of non-occupational exposure: Transmission of HIV can be prevented through use of condoms and safer drug injection techniques. HIV infection can be prevented with use of antiretroviral medications as PrEP to protect an individual who engages in behaviors that may result in exposure to HIV. “Treatment as prevention (TasP)” and “undetectable equals untransmittable (U=U)” are evidence-based strategies for greatly reducing the risk of HIV transmission through sexual exposure.
Sexual Assault Exposure Risk
Statistics on sexual assault in the United States show high rates of attempted or completed rape among several populations, including cisgender women, men, children, and transgender individuals:
- 21.3% of women reported attempted or completed rape* in their lifetime, with the first assault occurring Smith, et al. 2018:
- Before age 18 years in 43.2% (~11 million)
- Between the ages of 11 and 17 years in 30.5% (~7.8 million)
- At age 10 or younger in 12.7% (~3.2 million)
- 1.4% of men reported attempted or completed rape in their lifetime, with their first experience1 occurring Smith, et al. 2018:
- Before age 18 years in 26% (~2 million)
- Between the ages of 11 and 17 years in 19.2% (~1.5 million)
- 26% of women and 15% of men who were victims of sexual violence, physical violence, or stalking by an intimate partner in their lifetime first experienced these or other forms of violence by that partner before age 18 years CDC 2023.
- 10% of 27,715 respondents to the 2015 U.S. Transgender Survey reported that they had been sexually assaulted in the 12 months prior to survey completion; 47% reported that they had experienced sexual assault during the course of their lives. James, et al. 2016.
*See How NISVS Measured Sexual Violence for definitions.
Risk of HIV infection: Increased risk of infection in cases of sexual assault has been associated with trauma at the site of exposure and absence of barrier protection:
- Genitorectal trauma has been documented in 50% to 85% of sexual assault patients Sachs and Chu 2002; Jones, et al. 2009; Sommers, et al. 2012, and anogenital trauma has been observed in 20% to 85% Riggs, et al. 2000; Grossin, et al. 2003; Jones, et al. 2003; Sugar, et al. 2004; Laitinen, et al. 2013; Larsen, et al. 2015.
- High rates of unprotected receptive anal intercourse (88%) and vaginal penetration (>60%) have been reported Draughon Moret, et al. 2016. Perpetrators of intimate partner violence are not likely to use condoms (or use condoms inconsistently), are likely to force sexual intercourse without a condom and to have sexual intercourse with other partners Raj, et al. 2006; Casey, et al. 2016; Stephenson and Finneran 2017.
PEP is the only proven method of reducing HIV acquisition after exposure, and it should be offered in cases of sexual assault. There are published reports of HIV seroconversion following sexual assault Murphy, et al. 1989; Claydon, et al. 1991; Albert, et al. 1994; Myles, et al. 2000.
Exposure Risk in Children
Although there is evidence to support HIV prophylaxis for perinatal exposure, there are no randomized clinical trials of PEP in children beyond the perinatal period. Types of exposures that may be reported in children include sexual assault, needlesticks, or bite from a child who has HIV, but as noted below, this last type of exposure is no longer likely to occur.
Biting: Biting is a common occurrence among young children and in daycare settings. The levels of HIV detected in saliva alone are very low. The few documented cases of possible HIV transmission following bites occurred in adults exposed to blood-tinged saliva Vidmar, et al. 1996; Pretty, et al. 1999; Andreo, et al. 2004. As mentioned previously, a recent systematic review found no cases of HIV transmission through spitting and 9 possible cases of transmission through biting Cresswell, et al. 2018. A bite is not considered a risk exposure to either party when the integrity of the skin is not disrupted. Because there are so few children with HIV now, it is unlikely that a child would be the source of an HIV exposure.
Sexual abuse: HIV transmission has been described in children who have been sexually abused, and this abuse was identified as the only risk factor for infection Gellert, et al. 1993; Lindegren, et al. 1998. Children might be at increased risk of becoming infected with HIV due to the cervical ectopy in adolescent girls and to the thinness of the vaginal epithelium in prepubertal girls Kleppa, et al. 2015. In addition, children who experience abuse multiple times over an extended period by the same perpetrator are at increased risk due to mucosal trauma with bleeding Dominguez 2000; Smith, et al. 2005; CDC 2016.
Discarded needles: Risk of transmission from discarded needles is thought to be low. In 2 cohorts of children (1 with 59 children and the other with 249) exposed to needlesticks from discarded needles, there was no HIV transmission American Academy of Pediatrics 1999. HIV could not be isolated from the washings of 28 discarded needles from public places and 10 needles collected from a needle exchange program American Academy of Pediatrics 1999. In a Canadian study evaluating 274 pediatric community-acquired needlestick injuries, only 30% of those exposed received PEP, but there were no seroconversions in 189 children tested for HIV after 6 months Papenburg, et al. 2008. These studies, as well as the intolerance of HIV to environmental conditions through exposure to air over time, provide reassuring data regarding the low risk of transmission from this type of exposure. See Table 1: Baseline Testing Based on Age of Exposed Individual and Type of Exposure and Table 6: Recommended Monitoring After PEP Initiation for recommendations regarding laboratory testing, including for hepatitis C virus, based on type of exposure.
| KEY POINTS |
|
Rationale for PEP and Evidence of PEP Effectiveness
Post-exposure prophylaxis (PEP) has been established to effectively prevent HIV infection in an exposed individual when initiated within 2 hours (ideal) and no later than 72 hours after an exposure. Rapid and effective response to a reported HIV exposure are key to the successful prevention of HIV infection.
PEP blocks viral replication: After percutaneous or mucosal exposure to HIV, local replication of virus occurs in tissue macrophages or dendritic cells (see Figure 1, below). However, if infection cannot be contained at this stage, it is followed within 48 to 72 hours by replication of HIV in regional lymph nodes. Viremia then follows within 72 to 120 hours (3 to 5 days) of virus inoculation.
This sequence of events carries significant implications. Given the rapid appearance of productively-infected cells following the introduction of virus, PEP regimens with the most rapid onset of activity, multiple sites of antiviral action, and greatest potency are likely most effective.
| KEY POINTS |
|
Evidence of PEP effectiveness: Evidence of PEP effectiveness has been derived primarily from animal model studies and extrapolated from clinical trials of ARV prophylaxis to prevent perinatal transmission of HIV.
Evidence from animal models: Animal studies demonstrate time-dependent efficacy of PEP within 72 hours of exposure, with excellent efficacy reported if initiated within 36 hours Otten, et al. 2000; Tsai, et al. 1998.
- In a recent study, infected mice injected intraperitoneally with fluorescently labeled HIV-1 had no detectable plasma p24 or HIV-1 RNA when treated with raltegravir 1 day post infection. Ten mice that were not treated and became positive for plasma p24 and HIV-1 RNA and developed swollen lymph nodes in the peritoneal cavity Ogata-Aoki, et al. 2018.
- A systematic review and meta-analysis identified 16 studies that specifically assessed the efficacy of PEP (N = 180) compared with controls (N = 103). A pooled analysis of all animal studies reported the risk of seroconversion was 89% lower among primates exposed to PEP than among controls Irvine, et al. 2015.
- In macaques exposed to HIV intravaginally, PEP initiated at 12 and 36 hours post exposure prevented infection; however, breakthrough plasma viremia was observed in some animals when PEP was initiated 72 hours post exposure Otten, et al. 2000.
- SIV infection was prevented in macaques treated 24 hours post exposure with ARV medications as PEP (short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine); half of the subjects that received PEP at 48 and 72 hours post exposure developed infection Tsai, et al. 1998.
Figure 1: Sequence of Events Following HIV Exposure, With and Without Administration of PEP
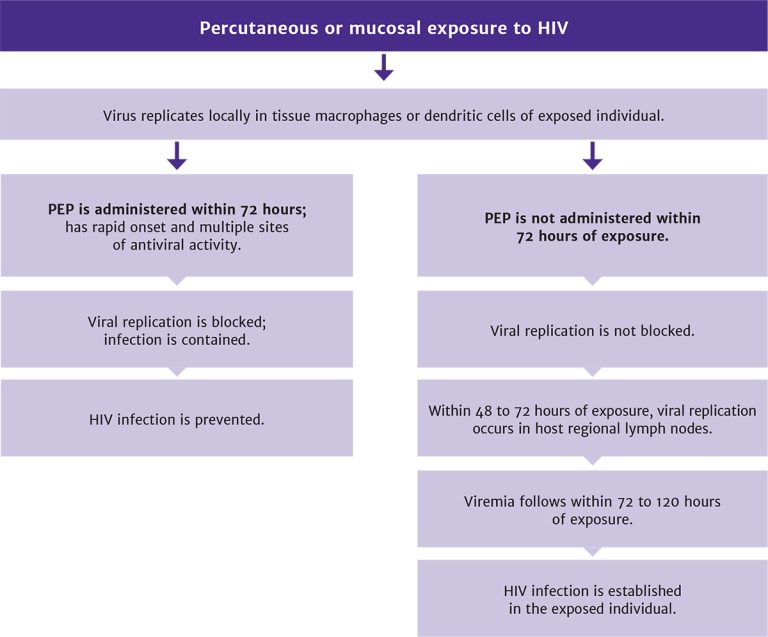
Download figure: Sequence of Events Following HIV Exposure, With and Without Administration of PEP
Evidence from human studies: A limited number of case-control studies and clinical trials have established PEP effectiveness in humans.
- Occupational exposure: In a Centers for Disease Control and Prevention (CDC) retrospective case-control study of zidovudine (ZDV) use after occupational HIV exposure in healthcare workers, the risk of HIV infection was reduced by 81% in those who received ZDV Cardo, et al. 1997. In a 4-country study, 33 cases of occupationally acquired HIV were compared with 665 control subjects. Case patients were significantly less likely than control subjects to have taken ZDV prophylaxis after exposure, with an odds ratio of 0.19 Cardo, et al. 1997.
- Since 1999, only 1 confirmed case of occupationally acquired HIV has been reported to the CDC Joyce, et al. 2015. In this case, a laboratory technician sustained a needle puncture while working with concentrated HIV cultures, which is a very high-risk scenario.
- PEP following needle sharing and transfusion: No specific studies currently address PEP use and its efficacy among individuals who inject drugs and share needles, and no data are currently available regarding HIV transmission via needle sharing when the source has an undetectable viral load.
- Retrospective analyses of PEP do include small numbers of participants with injection drug use as a risk factor and did not report PEP failures among this group McDougal, et al. 2014; Kahn, et al. 2001.
- One case report demonstrated PEP effectiveness for a 12-year-old girl with sickle cell disease who received 4-drug PEP with tenofovir, emtricitabine, ritonavir-boosted darunavir, and raltegravir after a blood transfusion and exposure to the blood of a donor who had an HIV viral load of 9,740 copies/mL Al-Hajjar, et al. 2014.
Evidence from studies of seroconversion with PEP use after sexual exposure: Observational cohorts have provided some data about seroconversion rates among PEP users and possible risk factors among seroconverters.
- A retrospective study analyzed all non-occupational PEP courses prompted by sexual exposure at a California health center to determine factors associated with seroconversion within 24 weeks of initiating PEP. The incidence rate of HIV infection was 2.3/100 person-years. Of note, 17 seroconversions occurred among 1,744 individuals who followed up within the 24-week period; of these 17 seroconversions, 7 had re-exposure risks, 8 had condom-protected sex only, and 2 reported abstinence from sex following the exposure for which they received PEP. In a multivariate analysis, significant predictors of seroconversion included methamphetamine use, incomplete PEP medication adherence, and time from initial exposure to PEP dose >48 hours but <72 hours Beymer, et al. 2017.
- One systematic review analyzed completion rates among 15 studies (1,830 initiations) of 2-drug PEP regimens and 10 studies (1,755 initiations) of 3-drug PEP regimens. Although the failure rate as determined by HIV seroconversion could not be compared because events overall were rare and protocols for follow-up were not uniform, the data underscore the value and effectiveness of PEP initiation Ford, et al. 2015.
PEP following sexual assault of children and adolescents: One study reported that in an inner-city pediatric emergency department in an area with high HIV prevalence, PEP was offered to 87 survivors of sexual assault who qualified for the intervention. Of those 87 children, only 5.7% were provided with PEP, but 69% were given antibiotic prophylaxis to prevent sexually transmitted infections other than HIV Fajman and Wright 2006. The reasons for such a low number (5 children) of PEP initiations were not provided. Among those who did receive PEP, there was no record of seroconversions, but 2 of those patients were lost to follow-up. The study had many limitations.
First Dose of PEP and Management of the Exposure Site
| RECOMMENDATIONS |
|
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis. Note:
|
Exposure to HIV Is an Emergency
An HIV exposure is a medical emergency and rapid initiation of PEP—ideally within 2 hours and no later than 72 hours post exposure—is essential to prevent infection. Therefore, this committee encourages emergency departments, outpatient clinics, and urgent care centers to train triage staff to assign high priority to patients who report a potential exposure. In deciding whether to continue PEP beyond the first emergency dose, care providers must balance the benefits and risks. PEP can be discontinued later in the evaluation process if indicated.
Because the efficacy of PEP in preventing an established HIV infection diminishes rapidly, initiation as soon as possible after exposure is best Kuhar, et al. 2013; CDC 2016. Animal models have consistently demonstrated improved outcomes at 12 to 36 hours post exposure compared with 72 hours Black 1997; Tsai, et al. 1998; Van Rompay, et al. 1998; Otten, et al. 2000; Smith, et al. 2000; Van Rompay, et al. 2000. Consistent with these findings, the virus can be detected in the regional lymph nodes of SIV-infected rhesus macaques within 2 days of intravaginal exposure Spira, et al. 1996.
| NEW YORK STATE LAW: MINOR CONSENT |
|
| KEY POINTS: TIME TO PROTECTION WITH PREP |
|
PEP for an individual who is taking PrEP: On occasion, an exposed individual who has been taking PrEP may insist on receiving a third ARV medication as PEP despite a clinician’s reassurance that it is not necessary. A clinician may reassure a patient who is taking PrEP with daily adherence that no current evidence can support adding an additional ARV after a potential exposure. However, if the exposed individual has only recently started taking PrEP, has been taking PrEP inconsistently, or has been taking the medications “on-demand,” it may be reasonable to consider a 28-day course of 3-drug PEP after a high-risk exposure. Similarly, if the source has virus with known underlying resistance to the components of a PrEP regimen (emtricitabine or tenofovir), offering 3-drug PEP to the exposed individual should be considered, particularly if the source’s viral load is not suppressed (i.e., <200 copies/mL). Lastly, there may be instances where the clinician may have to balance an exposed individual’s level of anxiety with maintaining the therapeutic alliance between the patient and care provider: offering 3-drug PEP in these scenarios may be appropriate to daily PrEP users in rare circumstances, such as high-risk needle sharing exposures or on a case-by-case basis. A request for PEP from a patient who is consistently using PrEP should not be accommodated following an exposure that is evaluated to be low or zero risk.
Request for PEP later than 72 hours post exposure: Because evidence indicates that PEP is not effective when initiated more than 72 hours post exposure, clinicians should not initiate PEP after this time point Black 1997; Tsai, et al. 1998; Van Rompay, et al. 1998; Otten, et al. 2000; Smith, et al. 2000; Van Rompay, et al. 2000; Beymer, et al. 2017.
| KEY POINT |
|
After 72 hours post exposure, HIV infection may have been established. If PEP is prescribed after 72 hours and then discontinued after 28 days, the risk of viral rebound with that inadvertent interruption in ART is significant, as is the associated risk of developing resistance to ART; therefore, this committee stresses that PEP should not be initiated later than 72 hours post exposure.
In response to an exposure reported after 72 hours post exposure, follow-up that is appropriate to the type of exposure should be arranged (see Table 1: Baseline Testing Based on Age of Exposed Individual and Type of Exposure):
Occupational exposure: Serial HIV testing, serial hepatitis C virus (HCV) testing, and hepatitis B virus (HBV) prophylaxis if indicated based on prior immunity status (e.g., records of HBV surface antibody titers).
Non-occupational exposure: Serial HIV testing, serial HCV testing, HBV prophylaxis if indicated, and appropriate screening for sexually transmitted infections (STIs). Provide risk-reduction counseling and linkage to PrEP services if indicated.
Sexual assault exposure: Serial HIV testing, serial HCV testing, HBV prophylaxis if indicated, empiric STI treatment, and linkage to appropriate services and support.
Exposure in a child aged 2 to 12 years: Serial HIV testing, HCV antibody testing, HBV prophylaxis if indicated, empiric STI treatment if sexual assault exposure, and linkage to appropriate services and support.
Note: See guideline section Management of Potential Exposure to Hepatitis B Virus for indications for HBV prophylaxis.
Management of the Exposed Site
Care of the exposure site should prioritize appropriate cleansing and infection preventive measures and minimize further trauma and irritation to the exposed wound site. The site of a wound or needlestick injury should be cleaned with soap and water only. It is best to avoid use of alcohol, hydrogen peroxide, povidone-iodine, or other chemical cleansers. Squeezing the wound may promote hyperemia and inflammation at the wound site, potentially increasing systemic exposure to HIV if present in the contaminating fluid. The use of surgical scrub brushes or other abrasive tools should be avoided, as they can cause further irritation and injury to the wound site. Eyes and other exposed mucous membranes should be flushed immediately with water or isotonic saline.
When to Consult an Expert Regarding the First Dose of PEP
Examples of clinical scenarios that warrant consultation with an experienced HIV care provider include: a source with ARV-resistant HIV, an exposed individual with limited options for PEP medications due to potential drug-drug interactions or comorbidities, or an exposed individual who is pregnant or unconscious.
Expert consultation for New York State clinicians: In such circumstances, clinicians are advised to call the Clinical Education Initiative (CEI Line) to speak with an experienced HIV care provider. Call 866-637-2342 and press “1” for HIV PEP. The CEI Line is available 24/7.
The Clinical Consultation Center (CCC) for PEP may be reached by calling 888-448-4911. The CCC is part of the AIDS Education and Training Centers and is located at the University of California, San Francisco/Zuckerberg San Francisco General Hospital. It is funded by the Health Resources and Services Administration and the Centers for Disease Control and Prevention (see UCSF > PEP for more information, including hours).
| SELECTED GOOD PRACTICE REMINDERS |
|
First Dose of PEP and Management of the Exposure Site
|
Exposure Risk Evaluation
| RECOMMENDATION |
All Exposures
Sexual Assault Exposure
|
Abbreviations: HBV, hepatitis B virus; HPV, human papillomavirus; PEP, post-exposure prophylaxis; STI, sexually transmitted infection. |
| Box 2: Risk of HIV Transmission From a Source With HIV | |
Meaningful risk of transmission:
|
No meaningful risk of transmission:
|
Occupational Exposure Risk Evaluation
PEP is indicated whenever an occupational exposure to blood, visibly bloody fluids, or other potentially infectious material occurs through percutaneous or mucocutaneous routes or through non-intact skin. Figure 2, below, illustrates the steps in determining whether ongoing PEP is indicated after the first emergency dose.
Occupational exposures for which PEP is indicated include the following:
- Break in the skin by a sharp object (including hollow-bore, solid-bore, and cutting needles or broken glassware) that has been in the source’s blood vessel or is contaminated with blood, visibly bloody fluid, or other potentially infectious material.
- Bite from a patient with visible bleeding in the mouth that causes bleeding in the exposed individual.
- PEP is not indicated for an exposure to saliva, including from being spat on, in the absence of visible blood.
- Splash of blood, visibly bloody fluid, or other potentially infectious material to the mouth, nose, or eyes.
- A non-intact skin (e.g., dermatitis, chapped skin, abrasion, or open wound) exposure to blood, visibly bloody fluid, or other potentially infectious material.
Evaluation for other bloodborne pathogens: See guideline sections Management of Potential Exposure to Hepatitis B Virus and Management of Potential Exposure to Hepatitis C Virus.
Figure 2: Occupational HIV Exposure: PEP and Exposure Management When Reported Within 72 Hours
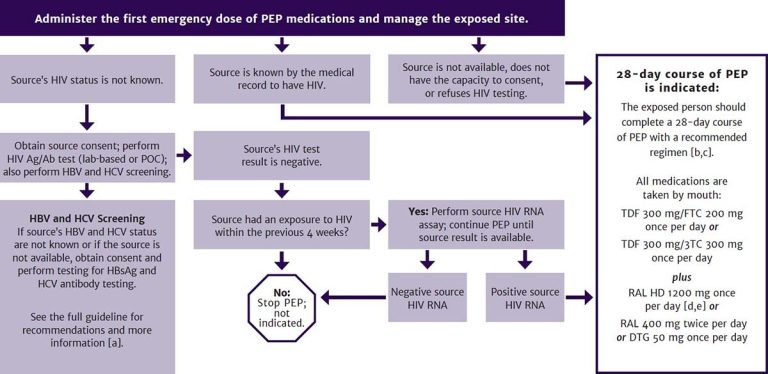
Abbreviations: Ag/Ab, antigen/antibody; CrCl, creatinine clearance; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PEP, post-exposure prophylaxis; POC, point-of-care.
Drug name abbreviations (brand name): 3TC, lamivudine (Epivir); DTG, dolutegravir (Tivicay); FTC, emtricitabine (Emtriva); RAL, raltegravir (Isentress); TDF, tenofovir disoproxil fumarate (Viread); TDF/FTC (Truvada).
Notes:
- For HBV and HCV post-exposure management, see guideline sections Management of Potential Exposure to Hepatitis B Virus and Management of Potential Exposure to Hepatitis C Virus.
- See Tables 2 and 3 for preferred and alternative PEP regimens.
- Do not use fixed-dose combination tablet for patients who require dose adjustment for renal failure. Adjust dose of TDF/FTC or TDF/3TC for patients with CrCl <50 mL/min (see NYSDOH AI guideline Selecting an Initial ART Regimen > ARV Dose Adjustments for Hepatic or Renal Impairment).
- RAL HD may be prescribed for patients who weigh >40 kg.
- RAL HD should not be prescribed for pregnant individuals.
Non-Occupational Exposure Risk Evaluation
In many cases of non-occupational exposure, the source is not available for testing. The HIV status of the source should not be the focus of the initial evaluation; determination of whether the exposure warrants PEP and, when indicated, prompt initiation of PEP, should be the focus. Figure 3, below, illustrates the steps in determining whether ongoing PEP is indicated after the first emergency dose.
| KEY POINTS |
|
Risk of transmission: Box 1: Risk per 10,000 Exposures of Acquiring HIV From an Infected Source and Factors That Increase Risk provides the risk of HIV infection following various types of non-occupational exposure to an individual known to have HIV and factors that may increase risk. HIV transmission occurs most frequently during sexual or drug use exposures; however, many factors can influence risk.
Exposure to a source with acute HIV: Due to the presence of high HIV viral load levels, the probability of transmission when the source is in the acute and early stage of HIV infection (first 6 months) is 8- to almost 12-fold higher than it is once a source’s viral set point has been established, typically about 6 months after infection Pilcher, et al. 2004; Wawer, et al. 2005. The presence of STIs in either the source or the exposed individual also increases risk of HIV transmission Advisory Committee for HIV and STD Prevention 1998; Johnson and Lewis 2008; CDC 2017. Conversely, transmission risk with sexual exposure is significantly decreased when a source is taking effective antiretroviral therapy (ART) and has an undetectable viral load Cohen, et al. 2011.
Box 3, below, lists non-occupational exposures that should prompt consideration of PEP and those that do not warrant PEP.
| Box 3: Non-Occupational Exposure Risks and Indications for PEP |
Higher-Risk: PEP Is Recommended:
Lower-Risk: Assess Factors That Increase Need for PEP:
PEP Is Not Indicated [b]:
|
|
Abbreviation: PEP, post-exposure prophylaxis. Notes:
|
A frank discussion between the clinician and an exposed individual regarding sexual activities, needle sharing, and other drug-using activities that have the potential for exposure to blood and other body fluids can help determine a patient’s need for PEP (see Box 1: Risk per 10,000 Exposures of Acquiring HIV From an Infected Source and Factors That Increase Risk and Box 3, above). The behaviors that confer the highest risk are needle sharing and receptive unprotected anal intercourse with an individual who has HIV DeGruttola, et al. 1989; CDC 1997; Varghese, et al. 2002.
Clinicians should also assess factors that have been associated with increased risk of HIV infection, including:
- Trauma at the site of exposure, especially if there was contact with blood, semen, or vaginal fluids.
- Presence of genital ulcer disease or other STIs LeGoff, et al. 2007; CDC 2017.
- High plasma viral load in a source with HIV Patterson, et al. 2002; Tovanabutra, et al. 2002.
- Exposure in an uncircumcised male Patterson, et al. 2002; Bailey, et al. 2007; Gray, et al. 2007.
Factors that may significantly decrease transmission of HIV include exposure to a source who is taking effective ART or use of daily PrEP and use of condoms during sexual exposures Weller and Davis 2002. After consensual sexual exposures that meet NYSDOH U=U Guidance criteria in the source, there is no evidence to support the use of PEP by the exposed individual. Furthermore, there is no evidence that a 3-drug PEP regimen provides any additional benefit to an exposed individual who adheres to a daily PrEP regimen; consistent use of PrEP has been shown to be 99% effective when taken appropriately. Correct condom use is highly effective in preventing transmission of HIV; however, during the post-exposure evaluation, it often is not possible to reliably ascertain whether condoms were used correctly or whether breakage, slippage, or spillage occurred.
Evaluation for exposure to STIs other than HIV: Risk behaviors leading to HIV infection also confer risk or exposure to other STIs. Patients who present for PEP after a consensual sexual exposure should be evaluated for other STIs.
Baseline testing generally cannot detect STIs that were acquired as a result of the exposure, but it may detect infections present prior to the exposure that prompted the evaluation for PEP. Presentation for PEP provides an opportunity to screen individuals at risk of STIs and treat infections as indicated. High rates of concomitant STIs at the time of presentation for PEP have been found in men who have sex with men Hamlyn, et al. 2006; Jamani, et al. 2013.
Routine empiric treatment for STIs is not recommended for consensual sexual exposures. Education about STI symptoms should be provided, and patients should be instructed to call their healthcare provider if symptoms occur. Follow-up STI screening should be considered at 2 weeks post exposure to definitively exclude STIs Mayer, et al. 2008; Tosini, et al. 2010; Mulka, et al. 2016; Mayer, et al. 2012; Oldenburg, et al. 2015; Mayer, et al. 2017; McAllister, et al. 2017.
Evaluation for other bloodborne pathogens: See guideline sections Management of Potential Exposure to Hepatitis B Virus and Management of Potential Exposure to Hepatitis C Virus.
Emergency contraception: For individuals who can but who do not desire to become pregnant, and who consent, emergency contraception should be initiated immediately. There are a range of methods (copper intrauterine device, levonorgestrel, and ulipristal acetate) that can be taken within 5 days of a sexual exposure. Of note, emergency contraception is not an abortifacient and will generally not disrupt an ongoing healthy pregnancy. For more information, see Bedsider: Emergency Contraception.
Figure 3: Non-Occupational HIV Exposure: PEP and Exposure Management When Reported Within 72 Hours

Abbreviations: Ag/Ab, antigen/antibody; CrCl, creatinine clearance; CT/GC NAAT, chlamydia/gonorrhea nucleic acid amplification testing; HBV, hepatitis B virus; HCV, hepatitis C virus; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Drug name abbreviations (brand name): DTG, dolutegravir (Tivicay); RAL, raltegravir (lsentress); TDF/3TC, tenofovir disoproxil fumarate/lamivudine (Cimduo); TDF/FTC, tenofovir disoproxil fumarate/emtricitabine (Truvada).
Notes:
- Do not use fixed-dose combination tablet if dose adjustment for renal failure is required. Adjust dose of TDF/FTC or TDF/3TC for patients with CrCl <50 ml/min (see NYSDOH AI guideline Selecting an Initial ART Regimen > ARV Dose Adjustments for Hepatic or Renal Impairment).
- For HBV and HCV post-exposure management, see guideline sections Management of Potential Exposure to Hepatitis B Virus and Management of Potential Exposure to Hepatitis C Virus.
Sexual Assault Exposure Risk Evaluation
The decision to recommend PEP to an individual who may have been exposed to HIV through sexual assault should not be based on the geographic location of the assault but rather on the nature of the exposure during the assault and the HIV status of the defendant, if known. Although the seroprevalence of HIV in different New York State communities may vary, the HIV status of an individual accused of sexual assault remains unknown until that individual has been tested.
| KEY POINTS |
|
Risk of HIV transmission: The risk of HIV transmission in sexual assault is greater due to the presence of genitorectal trauma, which may be present in as many as 50% to 85% of sexual assault patients Sachs and Chu 2002; Jones, et al. 2009; Sommers, et al. 2012. Studies on sexual assault document high rates of unprotected receptive anal intercourse (10% to 15%) and unprotected vaginal penetration (55% to 80%) Draughon Moret, et al. 2016. Studies also demonstrate a wide range (20% to 85%) of incidence of anogenital trauma Riggs, et al. 2000; Grossin, et al. 2003; Jones, et al. 2003; Sugar, et al. 2004; Laitinen, et al. 2013; Larsen, et al. 2015. In one study, 1% of men convicted of sexual assault in Rhode Island had HIV when entering prison Di Giovanni, et al. 1991, higher than the general male population (0.3%).
The absence of visible trauma does not rule out sexual assault; microabrasions and bruising are common, and the appearance of these manifestations following sexual assault may be delayed. Oral trauma may also occur during sexual assault, with potential exposure to blood, semen, or vaginal fluids from the defendant, which may carry a potential risk for HIV exposure. Bites or trauma may be inflicted during an assault and are indications for prophylaxis if there is the possibility of contact with blood, semen, or vaginal fluids from the defendant. A bite from a source with visible bleeding in the mouth that causes bleeding in the exposed individual is an indication for PEP.
HIV testing of the sexual assault patient should be performed in the emergency department setting. HIV testing may be performed on excess blood specimens obtained in the emergency department for other reasons, but only if informed consent has been obtained. In the absence of a baseline HIV test result, it may not be possible to establish that the assault resulted in HIV infection if the patient is later confirmed to have HIV.
If PEP is initiated, then responsibility for monitoring and follow-up should be coordinated by the treating clinician. If the baseline screening HIV test is reactive, then the assault patient should continue the PEP regimen until the result is confirmed with an HIV-1/HIV-2 antibody differentiation immunoassay or HIV RNA test and linkage to care with an experienced HIV care provider has been made. If the patient is not under the care of a primary care clinician, the emergency department clinician who has obtained the HIV test is responsible for ensuring that the patient is informed of the result promptly. If HIV infection has been diagnosed, the PEP regimen may be altered by the HIV care provider or continued in this case as ART.
Every hospital that provides emergency treatment to a sexual assault patient must adhere to and fully document services provided, consistent with the following standards of professional practice and Public Health Law 2805-P:
- Counsel sexual assault patients about options for emergency contraception to prevent pregnancy. Prompt access improves efficacy.
- Provide sexual assault patients with written information about emergency contraception that has been prepared or approved by the NYSDOH.
- Consider a urine pregnancy test to diagnose unplanned pregnancy, similar to STI screening in individuals who may be at risk. Inform the individual that a pregnancy test is being performed.
The following websites offer more information about the use of emergency contraception:
| NEW YORK STATE LAW |
|
STI prophylaxis: Clinicians should offer all sexual assault patients prophylactic medication to prevent gonorrheal and chlamydial infections and trichomoniasis. Rates of STIs have increased in all populations in the United States through a combination of increased incidence of infection and changes in diagnostic, screening, and reporting practices. Surveillance data for the United States indicate that between 2014 and 2018, rates increased for chlamydia (by 19%), gonorrhea (by 64%), primary and secondary syphilis (by 71%) and congenital syphilis(by 185%) CDC 2018; CDC(b) 2019. Trichomoniasis can be diagnosed or excluded in the emergency department if microscopy is available; otherwise, empiric treatment should be administered.
In cases of sexual assault, routine testing for gonorrhea, chlamydia, and syphilis is not recommended because test results would only determine whether the patient had an STI prior to the assault, and this information can be used to bias a jury against a survivor of sexual assault in court NYSDOH 2023.
Evaluation for exposure to other bloodborne pathogens: See guideline sections Management of Potential Exposure to Hepatitis B Virus and Management of Potential Exposure to Hepatitis C Virus.
Figure 4, below, illustrates the steps in determining whether ongoing PEP is indicated after the first emergency dose.
Figure 4: Sexual Assault HIV Exposure: PEP and Exposure Management When Reported Within 72 Hours
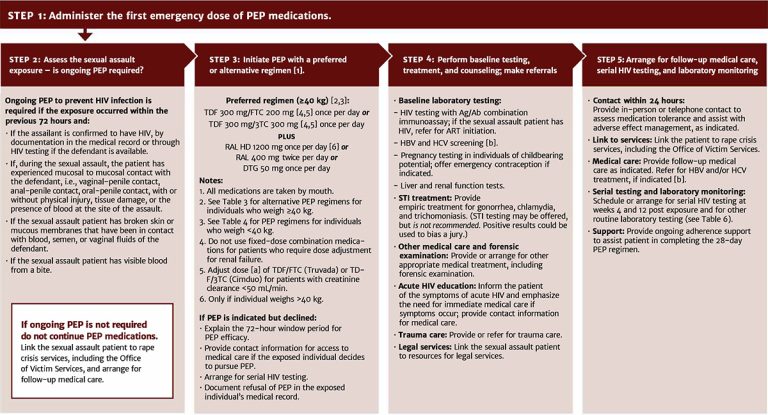
Abbreviations: Ag/Ab, antigen/antibody; ART, antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; PEP, post-exposure prophylaxis; STI, sexually transmitted infection.
Drug name abbreviations (brand name): 3TC, lamivudine (Epivir); DTG, dolutegravir (Tivicay); FTC, emtricitabine (Emtriva); RAL, raltegravir (lsentress); TDF, tenofovir disoproxil fumarate (Viread).
Notes:
- Do not use fixed-dose combination tablet for patients who require dose adjustment for renal failure. Adjust dose of TDF/FTC or TDF/3TC for patients with creatinine clearance <50 ml/min (see NYSDOH AI guideline Selecting an Initial ART Regimen > ARV Dose Adjustments for Hepatic or Renal Impairment).
- For HBV and HCV post-exposure management, see guideline sections Management of Potential Exposure to Hepatitis B Virus and Management of Potential Exposure to Hepatitis C Virus.
Considerations for Sexual Assault in Children
Care providers with experience in managing childhood sexual assault should assist in evaluating children who have been sexually assaulted to best assess the comprehensive needs of the child. Clinicians should assess children who are sexually assaulted for possible exposure to other STIs, including gonorrhea, syphilis, chlamydia, hepatitis B, hepatitis C, and trichomoniasis. Indications for laboratory evaluation and antimicrobial prophylaxis depend on the nature of the assault.
Once the initial, emergency dose of PEP has been administered, care for children exposed to HIV through sexual assault should be managed by a multidisciplinary team that includes the following:
- Clinicians with expertise in providing care for children who have been sexually assaulted
- Child protective services, which are mandated by law to conduct an initial assessment and investigation of reported assault/abuse
- Law enforcement officials to gather and evaluate evidence
- Rape crisis counselors or advocates to provide support to the child and family
- Mental health workers to provide immediate services as needed and who can provide long-term follow-up of the child and family, if appropriate
For more information, see The New York State Child Abuse Medical Provider Program > Education for Child Abuse Medical Providers.
Children who are sexually assaulted should be managed in an emergency department or other setting where appropriate resources are available to address the resulting medical, psychological, and legal issues. Children who present for care following sexual assault may have been victims of multiple exposures over time. PEP is indicated only for a sexual exposure that occurred within the 72 hours prior to the report of sexual assault. However, HIV testing may be indicated if a high-risk exposure occurred after the 72-hour cut-off for PEP efficacy.
For children who may have been exposed to HIV through sexual assault, the decision to continue PEP beyond the first emergency dose should be made based on the exposure evaluation; all sources of sexual exposure in children should be assumed to have HIV unless and until negative status can be confirmed. Clinicians should not delay initiating PEP in an exposed child pending results of the source’s HIV test.
| KEY POINTS: SEXUAL ASSAULT IN CHILDREN |
|
| SELECTED GOOD PRACTICE REMINDERS |
Exposure Risk Evaluation
|
Source HIV Status and Management
In many cases of non-occupational exposure, the source is not available for testing. The HIV status of the source should not be the focus of the initial evaluation; determination of whether the exposure warrants PEP and, when indicated, prompt initiation of PEP, should be the focus.
| RECOMMENDATIONS |
High-Risk Exposure
Continue PEP Until Source’s HIV Status Is Confirmed
If the Source Is Known to Have HIV
Nonreactive HIV Test Result in Source
|
Abbreviations: Ab, antibody; ART, antiretroviral therapy; ARV, antiretroviral; PEP, post-exposure prophylaxis. |
If the source is NOT available: When the source of any potential exposure to HIV is not known, not available, or cannot be HIV tested for any reason, the care provider should assess the exposed individual’s level of risk, assume the source has HIV until proven otherwise, and respond accordingly.
Determining whether the exposure warrants PEP and promptly initiating PEP when indicated should be the focus at initial presentation, rather than the HIV status of the source.
If the source IS available–test for HIV: When the source is available and consents to HIV testing, use of an HIV-1/2 antigen (Ag)/Ab combination immunoassay is recommended, preferably with a fast turn-around time. Results from point-of-care (POC) assays are available in less than 1 hour, and results from laboratory-based screening tests are often available within 1 to 2 hours. Rapid oral testing is not recommended due to lack of sensitivity to identify recent infection and requirements regarding food, drink, and tobacco use.
| Abbreviations: ART, antiretroviral therapy; PEP, post-exposure prophylaxis. | ||
| Box 4: Source HIV Testing | ||
Available Source with Confirmed HIV
|
Available Source with Unknown HIV Status
|
Unknown or Unavailable Source
|
| KEY POINTS |
|
When obtaining HIV testing in the source of a potential HIV exposure, consideration must be given to the source’s risk of HIV acquisition in the 4 weeks prior. During this period, often referred to as the “window period” of the HIV-1/2 Ag/Ab combination immunoassay, an initial HIV screening test may be nonreactive. If the source has engaged in condomless sexual intercourse (insertive or receptive anal, penile-vaginal) with or without pre-exposure prophylaxis (PrEP), or has shared intravenous needles or syringes with or without PrEP, then the source should also be tested for acute HIV infection with an HIV-1 RNA assay (qualitative or quantitative). Please note, only the qualitative HIV-1 RNA assay is U.S. Food and Drug Administration (FDA)-approved for aid in diagnosis of HIV.
PEP initiation should not be delayed; the first dose of PEP medications should be administered to the exposed individual before HIV testing and exposure evaluation. Only after the first dose of PEP has been administered should the source’s HIV serostatus, HIV exposure history, and other HIV-related information be evaluated to determine whether to continue PEP.
The most sensitive screening tests available should be used to allow for detection of early or acute HIV infection. The Centers for Disease Control and Prevention (CDC) and this Committee recommend screening with an FDA-approved Ag/Ab combination immunoassay, followed by confirmation with an FDA-approved HIV-1/HIV-2 Ab differentiation immunoassay. For more information, see the NYSDOH AI guideline HIV Testing and CDC/American Public Health Laboratories (APHL) Laboratory Testing Algorithm in Serum/Plasma.
Source with confirmed HIV: If the source is known to have HIV, information about their viral load, ART medication history, and history of ART drug resistance should be obtained, when possible, to assist in the selection of a regimen if PEP is indicated Beltrami, et al. 2003. Administration of the exposed individual’s first emergency dose of PEP should not be delayed while awaiting this information.
When a sexual exposure to a source with HIV occurs, the exposed individual may discontinue PEP if the source is taking ART and has an undetectable viral load at the time of exposure. In that scenario, providing information about U=U to the exposed individual and may be reassuring. However, if an exposed individual requests PEP, it should not be denied.
Informed consent: If the source is available and has an unconfirmed HIV status, then consent for voluntary HIV testing should be sought as soon as possible after the exposure. Clinicians should follow individual institutional policies for obtaining consent for HIV testing of the source. In New York State, when the source has the capacity to consent to HIV testing, that individual should be informed that HIV testing will be performed unless the source objects.
If the source objects, the care provider should inform the source that an HIV exposure may require the exposed individual to take medications to prevent infection, and the results of the source’s HIV test could help determine the duration of the exposed individual’s treatment. This information may encourage the source to agree to testing. However, if the source continues to refuse, then HIV testing cannot be performed.
| Box 5: Clinician-to-Clinician Communication |
Occupational exposure: Communication between clinicians is allowed; source information may be shared. Non-occupational exposure: Source information may be shared only if the source signs an Authorization for Release of Health Information and Confidential HIV-Related Information form DOH-2557. Sexual assault exposure: As of November 1, 2007, New York State Criminal Procedure Law § 210.16 requires HIV testing of criminal defendants indicted for certain felony sex offenses when requested by the individual who was assaulted. For guidance on defendant testing, see New York State Court-Ordered HIV Testing of Defendants. Exposure in a child: Source information may be shared only if the source signs an Authorization for Release of Health Information and Confidential HIV-Related Information form DOH-2557. |
HIV testing in the source of an occupational exposure: If a source does not have the capacity to consent, consent may be obtained from a surrogate, or anonymous testing may be performed if a surrogate is not immediately available (see Box 6, below). Clinicians should follow individual institutional policies for obtaining consent.
| Box 6: HIV Testing When the Source of an Occupational Exposure Is Unable to Consent |
The Family Health Care Decisions Act (FHCDA) stipulates who is able to consent for care. If a source is unable to provide consent for HIV testing, then clinicians should follow institutional policies related to the FHCDA for obtaining consent for the source’s HIV test. If the source is deceased, then anonymous testing should be performed. Healthcare proxy and other surrogacy status ends with death. No surrogate is immediately available to consent on behalf of the source: In cases of occupational exposures in which there is significant risk of contracting or transmitting HIV infection, an anonymous HIV test may be ordered without consent of the source if all 4 of the conditions listed below are met. Expeditious decisions regarding PEP for occupational exposures are essential. The decision to perform anonymous HIV testing of a source may be made immediately if no surrogate is present to provide consent.
Anonymous testing of the source: New York State public health law now allows healthcare providers to order anonymous testing in specific types of occupational exposures, and laboratories are no longer required to have a patient name to perform an HIV test in these cases. A clinician may order an anonymous HIV test only when an occupational exposure involves a source who is deceased, comatose, or otherwise unable to consent and there is no surrogate immediately available. The medical benefit of knowing the source’s test result must be documented in the exposed individual’s medical record. The result may not be documented in the source’s medical record. The result of the source’s anonymous HIV test is provided to the clinician providing care for the exposed worker for purposes of making decisions regarding PEP. Patient written authorization for release is not required. |
|
Source: NYSDOH AI Occupational Exposure and HIV Testing: Fact Sheet and Frequently Asked Questions |
| KEY POINT |
|
HIV testing in the source of a non-occupational exposure when the source is taking PrEP: If the source is taking PrEP, then plasma HIV RNA testing should be performed if the HIV-1/2 Ag/Ab combination immunoassay is negative, as is recommended for other groups at high risk (such as a source who reports possible exposure to HIV within the previous 4 weeks through sex or needle sharing). A negative viral load test will provide reassurance that the source is adherent to PrEP and allow the clinician and the exposed individual to rely on more than just the verbal report of the source.
HIV testing in the source of a sexual assault exposure: In most instances, the HIV status of the assailant will not be known and cannot be available in sufficient time to influence the decision to initiate PEP. If the HIV status of the defendant is established and confirmed, that knowledge should guide the decision to initiate or continue PEP; if the drug resistance data are available for a defendant with HIV, then that information can be used to tailor the PEP regimen. A negative HIV status of a defendant can determine whether the sexual assault patient should complete the 28-day PEP regimen; discontinuing unnecessary PEP has medical and psychological benefits. For more information, see NYSDOH Guidance for HIV Testing of Sexual Assault Defendants.
As of November 1, 2007, New York State Criminal Procedure Law § 210.16 requires testing of criminal defendants indicted for certain felony sex offenses for HIV, upon the request of the victim. For guidance on defendant testing, see NYS Court-Ordered HIV Testing of Defendants. Information regarding interpretation of HIV tests can be found in the CDC/APHL Laboratory Testing Algorithm in Serum/Plasma.
The increased risk of HIV transmission can be attributed to risk behavior profiles of the defendant, who engage in high-risk behaviors Klot, et al. 2013.
Confirmed defendant HIV status: If the defendant is confirmed to have HIV, then information about the defendant’s viral load, ART medication history, and history of ART drug resistance should be obtained, if possible, to assist in selection of a PEP regimen Beltrami, et al. 2003. Administration of the first emergency dose of PEP should not be delayed while awaiting this information.
HIV status of defendant is unknown or unconfirmed: Even if the individual reporting sexual assault knows the defendant, assumptions about HIV status or risk should have limited influence on the decision to initiate PEP. Familiarity with the defendant may influence the patient’s perception of risk and their decision to accept PEP. Because HIV risk behaviors and status may be hidden from close friends and family, decisions based on familiarity with the defendant should be made cautiously. It is not possible to know whether a defendant has HIV infection solely by risk behaviors. Categorical judgments should not be made on perceived risk. The decision to offer PEP should be based on whether significant exposure has occurred during the assault rather than on the risk behavior of the defendant.
| SELECTED GOOD PRACTICE REMINDERS |
Source HIV Status and Management
|
Baseline Testing of the Exposed Individual
| RECOMMENDATIONS |
All Exposures
Baseline STI Testing in Children
|
Abbreviations: Ab, antibody; Ag, antigen; ART, antiretroviral therapy; FDA, U.S. Food and Drug Administration; PEP, post-exposure prophylaxis; STI, sexually transmitted infection. |
Baseline HIV testing of the exposed individual identifies individuals who were already infected with HIV at the time of presentation (see Table 1, below). Results may inform decision-making regarding initiation of ART as treatment for established infection or initiation of 28 days of PEP to prevent HIV infection.
An initial reactive screening result must be confirmed with an HIV Ab differentiation immunoassay, and the PEP regimen should be continued until that result is obtained. Furthermore, the PEP regimen should be continued as rapid ART initiation if the reactive result is confirmed with an Ab differentiation immunoassay or HIV-1 RNA test, and the exposed individual should be referred to an experienced HIV care provider.
| KEY POINTS |
|
Exposed Workers
In cases of occupational exposure, exposed workers should be counseled that it is in their best interest to receive a baseline HIV test to document their HIV status at the time of the exposure. In the rare event of seroconversion following an occupational exposure, a negative baseline test is the only way to show that the exposed worker was infected as a result of the exposure.
Baseline HIV testing of the exposed worker is also used to identify individuals who were infected with HIV at the time of the exposure. This allows decisions to be made regarding the continuation of ART. If the baseline screening HIV test is reactive, then the exposed worker should continue the PEP regimen until the result is confirmed with an HIV-1/HIV-2 Ab differentiation immunoassay or HIV-1 RNA test and linkage to an HIV care provider has been established.
Individuals who decline baseline HIV testing risk the possibility of treatment interruption should they initiate PEP and refuse HIV baseline testing. However, refusal of baseline testing should not be a reason to withhold PEP in the event that an exposed worker had a high-risk exposure that warrants a 28-day course of PEP. Furthermore, the clinician should allow for testing to be performed within 3 days of PEP initiation to allow the exposed worker the opportunity to make an informed decision and to accommodate any anxiety or stress related to a possible HIV exposure.
Baseline Testing of Exposed Individuals
| Abbreviation: HCV, hepatitis C virus; NAAT, nucleic acid amplification test.
Note: In cases of non-sexual exposure in children aged 2 to 12 years, the medical record should be checked for history of tetanus vaccination.
|
|
| Table 1: Baseline Testing Based on Age of Exposed Individual and Type of Exposure | |
| Test | Age of Exposed Individual and Exposure Type |
| HIV-1/2 antigen/antibody combination immunoassay (HIV RNA testing may be required in some cases and within 72 hours in some cases) |
|
| Serum liver enzymes, blood urea nitrogen, creatinine |
|
| Complete blood count |
|
| Pregnancy (individuals of childbearing capacity) |
|
| Hepatitis B serology panel (surface antigen, surface antibody) |
|
| HCV antibody |
|
| Rapid plasma reagin (RPR) |
|
| Gonorrhea/chlamydia NAAT, by site |
|
| Trichomonas NAAT |
|
| SELECTED GOOD PRACTICE REMINDERS |
Baseline Testing of the Exposed Individual
Baseline Testing Following Sexual Assault Exposures
|
Selecting and Initiating a 28-Day Course of PEP
| RECOMMENDATIONS |
Preferred Regimens [a]
ARV Medications to Avoid for PEP
PEP During Pregnancy or Breastfeeding
|
Abbreviations: ARV, antiretroviral; CrCl, creatinine clearance; INSTI, integrase strand transfer inhibitor; PEP, post-exposure prophylaxis; PI, protease inhibitor. Notes:
|
Considerations and Caveats
Suspected seroconversion: If acute HIV infection is suspected at any time, immediate consultation with a clinician experienced in managing acute HIV infection is advised. Clinicians can call the Clinical Education Initiative (CEI Line) to speak with an experienced HIV care provider: 866-637-2342 (press “1” for HIV PEP). The CEI Line is available 24/7.
Source confirmed HIV negative: If the source is confirmed to be HIV negative, the exposed individual’s PEP regimen should be discontinued.
Use of a 3-drug PEP regimen: This committee recommends a 3-drug ARV regimen as the preferred option once the decision has been made to initiate PEP. When the source is known to have HIV, past and current ARV experience, viral load data, and genotypic or phenotypic resistance data (if available) may indicate the use of an alternative PEP regimen. Consult with an experienced HIV care provider.
Drug-drug interactions and adverse effects: Care providers should advise patients not to take divalent cations (aluminum, calcium, magnesium) or iron supplements concurrently with DTG or RAL. Metformin dosing should be limited to 1 g by mouth per day when an individual is taking DTG concurrently.
Care providers should counsel patients about the low risk of gastrointestinal adverse effects with TDF/FTC, such as nausea, abdominal bloating, and vomiting, along with headache. A low risk of neuropsychiatric effects with DTG may also exist. RAL has been rarely associated with rhabdomyolysis FDA 2013.
| RESOURCES: DRUG-DRUG INTERACTIONS INFORMATION |
Impaired renal function: Exposed individuals who have impaired renal function may require dose adjustments of ARV medications used for PEP and may require additional monitoring while completing a 28-day course of PEP DHHS 2023.
Hepatitis B virus infection: Additional monitoring is required for exposed individuals who have HBV infection.
Tenofovir alafenamide (TAF): Recommended and alternative regimens do not include TAF because evidence suggests decreased vaginal, cervical, and rectal tissue concentrations of the active form (tenofovir diphosphate) in healthy volunteers Cottrell, et al. 2017. This committee does not recommend including TAF in PEP regimens until further research is completed.
Adherence and completion requirements: The recommended 28-day treatment duration is based on limited animal data and expert opinion Tsai, et al. 1998. Nonetheless, adherence to a full 28-day course of PEP and completion of therapy is important to prevent HIV seroconversion post exposure.
Repeated requests for non-occupational PEP: PEP should not be routinely dismissed solely based on repeated risk behavior or repeat presentation for PEP (see guideline section Counseling and Patient Education > Risk reduction).
PEP completion following sexual assault: Limited data exist on the use of antiretroviral therapy (ART) to prevent HIV infection in sexual assault populations. One study demonstrated higher completion rates (66% vs. 42%) among individuals taking TDF/FTC in combination with DTG or RAL, as compared with those taking TDF/FTC plus darunavir (DRV) boosted with ritonavir (RTV) Kumar, et al. 2017, suggesting these regimens are better tolerated in this population.
| SELECTED GOOD PRACTICE REMINDERS |
Selecting and Initiating a 28-Day Course of PEP
|
Preferred PEP Regimens for Patients Who Weigh ≥40 kg
The medications that comprise the recommended PEP regimens (and substitutions) listed in Table 2, below, have favorable adverse effect profiles, fewer potential drug-drug interactions, and expected efficacy similar to older PEP regimens that contained ZDV or PIs. Researchers have reported increased rates of adherence and regimen completion when TDF/FTC or TDF/3TC have been used as components of the PEP regimen Mayer, et al. 2008; Tosini, et al. 2010. Observational cohorts and 1 small randomized study reported improved tolerability with TDF/FTC plus RAL Mulka, et al. 2016; Mayer, et al. 2012; McAllister, et al. 2017. Additionally, TDF/FTC has been highly successful in recent studies of pre-exposure prophylaxis Grant, et al. 2010; Baeten, et al. 2012; Thigpen, et al. 2012. One observational cohort demonstrated high completion rates with TDF/FTC plus DTG McAllister, et al. 2017.
Unlike PIs, which block HIV replication after integration with cellular DNA, all currently recommended medications (TDF/FTC plus DTG or RAL) act before viral integration with cellular DNA, providing a theoretical advantage in preventing establishment of HIV infection.
| KEY POINT |
|
| Abbreviations: CrCl, creatinine clearance; PEP, post-exposure prophylaxis.
Notes:
|
|
| Table 2: Preferred PEP Regimen for Patients Who Weigh ≥40 kg [a,b] | |
| Preferred Regimen | Notes |
plus
|
|
Download Table 2: Preferred PEP Regimen for Patients Who Weigh ≥40 kg Printable PDF
Alternative PEP Regimens for Patients Who Weigh ≥40 kg
Table 3, below, lists 2 alternative PEP regimens that are acceptable options when a preferred regimen is not available. They are possibly less well tolerated than the preferred regimen of TDF/FTC plus RAL or DTG, but they are significantly better tolerated than regimens containing ZDV or lopinavir/ritonavir (LPV/RTV). Observational studies have demonstrated excellent tolerability and completion rates Fätkenheuer, et al. 2016; Valin, et al. 2016; Mayer, et al. 2017.
A single-tablet regimen for a patient with adequate kidney function (CrCl >70 mL/min) and no expected drug-drug interactions may be a good option for those who prefer a once-daily, single-tablet PEP regimen. It also allows use of medication assistance programs if a patient has limited medication coverage options.
Drug-drug interactions: The potential for drug-drug interactions in patients receiving PIs or cobicistat (COBI) is increased due to the extensive cytochrome P450 interactions. Clinicians should assess for potential interactions before prescribing a PEP regimen.
| Abbreviations: CrCl, creatinine clearance; PEP, post-exposure prophylaxis.
Notes:
|
|
| Table 3: Alternative PEP Regimens for Patients Who Weigh ≥40 kg [a,b] | |
| Alternative Regimens | Notes |
|
For individuals with CrCl <70 mL/min: Fixed-dose single tablet EVG/COBI/TDF/FTC is contraindicated. |
|
For individuals with baseline CrCl <50 mL/min: Adjust dosing of 3TC/FTC plus TDF. |
Download Table 3: Alternative PEP Regimens for Patients Who Weigh ≥40 kg Printable PDF
| KEY POINT |
|
Other alternative PEP regimens: Other alternative PEP regimens may be acceptable in certain situations. Some clinicians continue to favor the use of ZDV in PEP regimens based on the results of a retrospective study supporting the efficacy of the agent Cardo, et al. 1997 and from long-term experience in occupational PEP. Clinicians who continue to prescribe ZDV should recognize and inform patients that the drug is associated with significant adverse effects and that better tolerated agents are available.
Use of LPV/RTV has greater potential for drug-drug interactions and adverse effects than RAL, DTG, or DRV/r (the preferred alternative boosted PI), with little added efficacy benefit expected. Studies have demonstrated decreasing PI resistance among HIV strains Paquet, et al. 2011, suggesting there may be a diminishing benefit to choosing LPV/RTV for its activity against resistant HIV strains. DRV/r has excellent activity against many PI-resistant strains and is better tolerated than LPV/RTV.
This committee recommends a 3-drug regimen because of the greater likelihood of enhanced effectiveness; however, if tolerability is a concern, use of a 2-drug regimen would be preferred to discontinuing the regimen completely. An early case-control study of occupational exposure demonstrated an 81% reduction in seroconversion with the use of ZDV monotherapy alone Cardo, et al. 1997, suggesting that treatment with any active ARV agent is beneficial in reducing risk. Other studies have investigated 2-drug PEP regimens and found excellent tolerability Mayer, et al. 2008; Kumar, et al. 2017.
PEP Regimens for Patients Who Weigh <40 kg
No clinical studies are available to determine the best regimens for HIV PEP in children. The recommendations for drug choices and dosages presented here follow current U.S. Department of Health and Human Services recommendations in Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection, which are based on expert opinion. The recommended regimens reflect experience with ARV combinations that effectively suppress viral replication in children with HIV and with combinations that are well tolerated and increase adherence to PEP. The chosen preferred regimens have demonstrated good potency and tolerability.
The alternative PEP regimens for children are also based on expert opinion. They all have demonstrated potent antiviral activity. However, the PI-containing regimens are often more difficult to tolerate, secondary to gastrointestinal adverse effects. To improve adherence, clinicians can and should prescribe preemptive antiemetics for anticipated gastrointestinal adverse effects.
When choosing a PEP regimen, care providers should consider factors that may affect adherence, such as ARV drug intolerance, regimen complexity, expense, and drug availability.
| Table 4: PEP Regimens for Patients 2 to 12 Years Old Who Weigh <40 kg [a] |
| See DHHS for dosing, administration, and additional information about each medication. Each medication name below is linked to a page about that medication.
Preferred: Tenofovir disoproxil fumarate (TDF; Viread) plus emtricitabine (FTC; Emtriva) plus raltegravir (RAL; Isentress). TDF/FTC is available as the fixed-dose combination (Truvada). Substitutions:
Alternatives:
|
Download Table 4: PEP Regimens for Patients 2 to 12 Years Old Who Weigh <40 kg Printable PDF
| KEY POINTS: SEXUAL ASSAULT IN CHILDREN |
|
ARV Medications to Avoid for PEP
Newer ARV medications have demonstrated significantly fewer adverse effects than older ARVs. The medications listed in Table 5, below, should be avoided.
| Table 5: Antiretroviral Medications to Avoid for Post-Exposure Prophylaxis | ||||
| ARV Class | Agent | <40 kg | ≥40 kg | Comments |
| First-generation protease inhibitors |
|
Avoid | Avoid | Poorly tolerated |
| First-generation non-nucleoside reverse transcriptase inhibitors |
|
Avoid | Avoid |
|
| Nucleoside reverse transcriptase inhibitors |
|
Avoid d4T, ddI, ABC, TAF | Avoid all |
|
| CCR5 antagonist | Maraviroc (MVC; Selzentry) | Avoid | Avoid | Only shows activity against R5-tropic virus |
Download Table 5: Antiretroviral Medications to Avoid for Post-Exposure Prophylaxis Printable PDF
Consultation with an experienced HIV care provider is recommended before using any of the medications listed above for PEP, or before using etravirine or doravirine, for which limited data exist.
PEP During Pregnancy or Breastfeeding
Use of ARV prophylaxis in pregnancy generally does not increase the risk of birth defects DHHS 2023. ARV prophylaxis can prevent HIV transmission during acute infection in pregnancy, when viral loads are extremely high, which is associated with a high risk of infection to the infant Patterson, et al. 2007. No severe adverse effects or adverse pregnancy outcomes have been noted among women taking ART for PEP CDC 2016. However, no clinical trial data regarding PEP use in pregnant individuals are currently available CDC 2016, and data are limited on the use of integrase inhibitors during pregnancy DHHS 2023.
When screening for HIV in pregnant patients, care providers should be aware that detection of early/acute HIV infection requires HIV RNA testing in most instances and should repeat antibody testing as late as the third trimester Wertz, et al. 2011 when screening for HIV infection in pregnant patients.
| KEY POINT |
|
Current U.S. Department of Health and Human Services guidelines require dose adjustments for DRV and atazanavir (ATV) DHHS 2023:
- DRV (Prezista): 600 mg twice per day plus RTV (Norvir) 100 mg twice per day
- ATV (Reyataz): 400 mg once per day plus RTV 100 mg once per day in the third trimester
Although birth defects and adverse effects on human fetuses have generally not been associated with the ARV agents that are currently available, exposure of a fetus to ARV agents during pregnancy carries a theoretical risk of embryotoxicity.
ARV medications to avoid as PEP during pregnancy: The ARV medications to be avoided for PEP above also apply to pregnant individuals. Based on animal data, there has been a theoretical concern for teratogenicity of EFV in the first trimester; however, current federal perinatal guidelines do not preclude its use DHHS 2023; Martinez de Tejada, et al. 2019. ZDV is still recommended for prevention of perinatal HIV transmission.
PEP during breastfeeding: Initiation of PEP in exposed individuals who are breastfeeding requires careful discussion. Both HIV and ARV medications may be found in breast milk; therefore, breastfeeding should be avoided for 3 months after the exposure to prevent HIV transmission and potential drug toxicities American Academy of Pediatrics 2013. Clinicians should discuss the risks and benefits with the patient. The infant’s pediatrician should be informed of any potential exposure to HIV or ARV medications.
Adherence and Completion of the 28-Day PEP Regimen
Reported adherence to a 28-day PEP regimen has historically been modest (40%-60%) Parkin, et al. 2000; Day, et al. 2006; Lunding, et al. 2010. However, increased rates of adherence have been reported in studies of PEP regimens that include TDF/FTC or TDF/3TC plus a third agent Mayer, et al. 2008; Tosini, et al. 2010, and some have reported improved tolerability with use of TDF/FTC plus DTG or RAL Mulka, et al. 2016; Mayer, et al. 2012; McAllister, et al. 2017.
Single-tablet regimens: With the availability of several single-tablet regimens, many clinicians prefer them for PEP to optimize adherence or to use commercial medication assistance programs that may be available to uninsured or under-insured individuals. Several recently published observational prospective cohort studies support this approach:
- Two recently published studies examined the use of fixed-dose TDF/FTC/elvitegravir (EVG)/COBI (Stribild) as PEP in observational prospective cohorts in France and Boston. In the French cohort, 92% of participants completed 28 days of PEP, and only 3 individuals switched to another regimen due to adverse effects Valin, et al. 2016. Lower rates of completion were noted in the Boston group, with 71% completing the 28-day course as prescribed (no missed doses), 15% stopping or modifying their dosing, and 14% lost to follow-up Mayer, et al. 2017. Both cohorts reported gastrointestinal adverse effects as the most common adverse events. Neither study documented HIV seroconversions.
- Results of a 2015 open-label, single-arm study conducted at 2 public sexual health clinics and 2 hospital emergency departments in Australia demonstrated high PEP completion rates (92%) and no HIV seroconversions with fixed-dose single tablet TDF/FTC/rilpivirine (RPV; Complera). Most participants (86%) reported taking all doses with food, and 95% of those who completed the full course endorsed taking the medication with food. The authors acknowledge that they studied TDF/FTC/RPV in a population with a low background of transmitted nucleoside reverse transcriptase inhibitor (NRTI) (4.1%) and non-NRTI (3.1%) resistance and that this combination should be used carefully in populations with higher rates of transmitted resistance Foster, et al. 2015.
The Centers for Disease Control and Prevention (CDC) and this committee recommend DTG as a third agent (and alternative to RAL). A recent open-label, single-arm study at 3 sexual health clinics and 2 emergency departments in Australia found completion rates of 90% and no seroconversions with use of DTG plus TDF/FTC as PEP. Adherence was 98%, measured by pill count and consistent with drug levels, and no unexpected adverse events or serious adverse events occurred McAllister, et al. 2017.
Alternatively, a once-daily PI-based PEP regimen of DRV/r plus 2 NRTIs has demonstrated lower discontinuation rates compared with LPV/r or EFV plus 2 NRTIs, without significant adverse events Fätkenheuer, et al. 2016. Together, these study results demonstrate that once-daily PEP regimens with multiple pills can be well tolerated and have high completion rates.
Regimens containing ZDV and LPV/r had lower rates of completion and higher rates of discontinuation due to adverse effects Ford, et al. 2015; Leal(a), et al. 2016. Many agency guidelines switched first-line recommendations to include RAL as a third agent because it had a more favorable adverse effect profile and fewer drug-drug interactions Mayer, et al. 2012; McAllister, et al. 2014. However, given the twice-daily dosing of RAL, nearly one-fourth of one cohort on PEP missed the afternoon dose Mayer, et al. 2012, which suggests that adherence to a RAL-based regimen is challenging.
Extending PEP Beyond 28 Days
It is rare that PEP is extended beyond the standard 28-day regimen. The only circumstances under which PEP would be extended include the following:
- The exposed individual has an indeterminate HIV test result at 4 weeks post exposure or is experiencing acute retroviral syndrome at 4 weeks post exposure.
- The exposed individual is pregnant and there is a high probability of HIV exposure, given the risk of viral rebound in pregnancy.
In these cases, the care provider should consult with an experienced HIV care provider. Otherwise, no data are available to support extending PEP beyond 28 days to prevent HIV infection following an exposure within the previous 28 days.
Counseling and Patient Education
The checklist in Box 7, below, includes topics for patient education for an individual exposed to HIV who has presented for post-exposure prophylaxis (PEP) or for the parent(s) or guardian(s) accompanying a child who is being evaluated for or initiated on PEP.
| Box 7: PEP Patient Education Checklist Address each item in clear, direct, easy-to-understand language and assess the individual’s comprehension of each topic before moving on. |
|
| Addressed and Understood: | |
| Reason for administering the first dose of PEP immediately. | |
| Process for evaluating the likelihood that the individual was exposed to HIV and the risk of infection. | |
| Use of PEP to help prevent HIV infection: Benefits, effectiveness, timing, and duration. | |
| Purpose of the HIV test and interpretation of results. | |
| Other baseline laboratory testing requirements and their purpose. | |
| What will happen if the exposed individual’s first HIV test is positive. | |
| If the source is available, what will happen if the source’s HIV test is positive. | |
| Follow-up visit and testing schedule and purpose. | |
| Possible drug-drug interactions: Evaluate the individual’s current medication list (e.g., prescription, over-the-counter, herbals, vitamins, supplements). | |
| How and when to take the PEP medications, including timing and food requirements. | |
Prescription for the additional 21 days of PEP: Where and when to get it filled and how to pay for the medications; provide information about sources of payment assistance if needed. See:
|
|
| Possible adverse effects and what to do if they occur. | |
Importance of adherence to the prescribed regimen:
|
|
| What to do if a dose of PEP is missed. | |
| Signs and symptoms of acute HIV infection and what to do if they occur. | |
| NEW YORK STATE LAW |
|
Information about serial HIV testing: Clinicians should educate the exposed individual about the “window period” of HIV infection and the importance of serial HIV testing to avoid a false-negative result during the early stages of infection. A negative baseline HIV test does not confirm negative status, so further testing at 4 and 12 weeks post exposure can determine seroconversion in any exposed individual, whether PEP is taken or not.
Clinicians should arrange appropriate medical follow-up for the exposed individual, particularly if an emergency department performed the initial evaluation and treatment. Appropriate medical follow-up includes access to a care provider in the event of possible PEP-related adverse effects or symptoms suggestive of acute retroviral syndrome (ARS). Toward that end, the exposed individual should be provided with a telephone number to reach an outpatient medical facility that can provide treatment within 24 hours to address adverse effects or to evaluate for ARS.
Symptoms of acute HIV infection: Inform exposed individuals about the possible symptoms of acute HIV:
- Influenza- or mononucleosis-like illness
- Fever and night sweats
- Lymphadenopathy
- Myalgias
- Arthralgias
- Sore throat
- Fatigue or malaise
- Headache
- Generalized rash
- Mucocutaneous ulcers
- Meningismus
- Oropharyngeal candidiasis
Because of the similarity of acute HIV infection to influenza- or mononucleosis-like illnesses, the exposed individual should be encouraged to seek medical attention if these symptoms develop, regardless of PEP use. The exposed individual should also be educated about the high risk of HIV transmission during acute HIV infection.
Adherence to the PEP regimen: Education about adherence should stress the need to take all doses of PEP medications as directed and to complete the 28 days of PEP unless otherwise directed. Make sure the patient understands that of a dose of PEP medications is missed, a “double-up” dose is not necessary. Instead, if dose is missed at a specific time, it can be taken as soon as it is remembered within 24 hours of the scheduled time.
Risk reduction: Individuals who present with potential HIV exposures as a result of ongoing engagement in risk behavior should be referred for pre-exposure prophylaxis (PrEP).
An individual’s intent to change behavior should be assessed, and an individualized risk-reduction plan should be developed. After completion of the 28-day PEP regimen, initiation of PrEP should be considered.
Occupational risk reduction: To decrease the risk of future exposures, employers are required to provide education regarding the prevention of needlestick injury at the time of hire and annually thereafter. Each institution should have internal protocols consistent with current state and federal laws.
Information for an exposed child and family: A potential HIV exposure in a child is likely to be an emotionally challenging situation for the family. Care providers should assess the health literacy of the parent(s) or guardian(s) and provide information at the appropriate level of understanding. Information should include risk of HIV acquisition based on type of exposure (see guideline section Risk of Infection Following an Exposure to HIV). This risk data may provide some reassuring perspective to the parent(s) or guardian(s). Emphasize that when PEP is initiated within the 72 hours following HIV exposure, failure is rare.
| RESOURCES |
| SELECTED GOOD PRACTICE REMINDERS |
Counseling and Patient Education
|
Providing PEP Medications and Other Services
| RECOMMENDATIONS: PROVIDING PEP MEDICATIONS AND OTHER SERVICES |
|
All exposures: If possible, clinicians should provide patients with a 28-day supply of post-exposure prophylaxis (PEP) medications. (A3) If a 28-day supply cannot be provided and if the patient does not have immediate access to a 28-day supply, then clinicians should provide a starter pack as indicated below. Occupational exposure: Clinicians should provide at least a 7-day starter pack of PEP medications to a worker assessed as having a high-risk exposure to HIV. (A3) Non-occupational exposure: Clinicians should provide a 7-day starter pack of PEP medications to an individual assessed as having a high-risk exposure to HIV. (A3) Sexual assault exposure: Clinicians are required by New York State law to provide a 7-day starter pack of PEP medications to sexual assault patients who are ≥18 years old and the full, 28-day course of PEP medications to those who are <18 years old. Sexual assault exposure in a child: Clinicians should provide 28 days of PEP medications to children (any individual <18 years old) who have been sexually assaulted and are assessed as having a high-risk exposure to HIV. (A3) Other types of high-risk exposures in children: Clinicians should provide a 7-day starter pack of PEP medications to a child assessed as having a high-risk exposure to HIV. If a child can take only liquid medications, then a 28-day supply should be provided. (A3) |
PEP Starter Pack
Starter packs may reduce the time to PEP initiation and have been used in several PEP protocols, including emergency department visits following sexual assault Krause, et al. 2014; Kumar, et al. 2017; Muriuki, et al. 2017. If a 28-day supply of medications cannot be provided, then in most cases, a 7-day supply will allow an individual sufficient time to access the additional medications needed to complete the full course of treatment. Patients who receive a 7-day starter pack should be informed that it does not contain the full 28-day course of PEP medication and assisted in creating a plan to obtain the rest of the required medications.
| KEY POINTS |
|
Payment For Occupational PEP
Federal law requires covered employers to ensure that all medical evaluations and procedures, vaccines, and post-exposure prophylaxis are made available to the employee within a reasonable time, at a reasonable location, and at no cost to the employee (OSHA, 1910.1030 Bloodborne Pathogens).
The New York Public Employee Safety Health Act (PESH) and Occupational Safety and Health Administration (OSHA)’s Bloodborne Pathogen Standards indicate that the covered employer is responsible for all costs associated with an exposure incident. An employer may not require any out-of-pocket expenditures on behalf of the employee, such as requiring the employee to utilize workers’ compensation if prepayment is required or compelling an employee to use health insurance to cover these expenses unless the employer pays all premiums and deductible costs associated with the employees’ health insurance.
Federal law: Federal law mandates that employers must ensure that all medical evaluations and procedures, vaccines, and PEP medications (7-day starter pack and access to the full 28-day course of medications) are made available to the employee within a reasonable time, at a reasonable location, and at no cost to the employee (OSHA, 1910.1030 Bloodborne Pathogens).
Employers should determine who will pay for PEP and establish policies for submitting claims to their workers’ compensation plans. Employers should not expect exposed workers to pay out of pocket for PEP, including copays, even if they are reimbursed at a later date.
Payment Assistance For Non-Occupational PEP
Care providers should ensure that a patient can acquire the medications needed to continue PEP through 28 days regardless of insurance coverage status. Options for patients who are uninsured or under-insured include medication assistance programs (MAPs) and health centers specifically funded to provide PEP at no or low cost.
If an individual has prescription drug coverage, third-party reimbursement may cover PEP, depending on the plan’s prescription drug policy. If a medication-dispensing facility does not receive reimbursement for these services, such expenses may be included in their annual Institutional Cost Report as part of indigent care costs. For patients who are paying out of pocket, cost is a factor in selecting a regimen.
| KEY POINT |
|
MAPs: MAPs are available for individuals who do not have insurance coverage for PEP and who meet certain criteria and cover several drugs included in the recommended PEP regimens:
- Fixed-dose tenofovir disoproxil/emtricitabine (TDF/FTC; Truvada)
- Dolutegravir (DTG; Tivicay)
- Raltegravir (RAL; Isentress)
- Single-tablet, fixed-dose elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (EVG/COBI/FTC/TDF; Stribild), a preferred alternative PEP regimen
Clinicians should work with social workers and support staff to enroll patients in these programs, if indicated, to provide PEP to patients without alternative means of coverage or payment. These programs often provide 1 course of PEP. Obtaining future courses may be challenging, so clinicians should consider whether pre-exposure prophylaxis is appropriate for patients who receive PEP from a MAP.
Payment for PEP Medications For Exposed Children
In New York State, all children qualify for health insurance regardless of their immigration status. Payment difficulties may arise for patients who have private insurance with high medication copays.
| RESOURCES: NYSDOH PEP PAYMENT OPTIONS |
|
Payment Methods For PEP Following Sexual Assault
Various methods of payment for PEP are available for victims of sexual assault, including Medicaid, Medicare, or the New York State (NYS) Office of Victim Services (OVS).
See NYSDOH Payment Options for Adults and Adolescents for PEP Following Sexual Assault.
Medication starter pack: Timely initiation of medication is crucial to the success of PEP, and amendments to Public Health Law section 2805-i and Executive Law section 631 effective June 15, 2020, require hospitals providing treatment to survivors of sexual assault to:
- Offer and make available a 7-day starter pack of HIV PEP to survivors of sexual assault who are ≥18 years old and
- Offer and make available the full 28-day supply of HIV PEP to survivors of sexual assault who are <18 years old.
Additionally, there are changes to hospital reimbursement for HIV PEP and sexual assault forensic exams. See the NYSDOH letter Dear Colleague HIV PEP Guidance Update for additional details.
| NEW YORK STATE LAW: PEP MEDICATIONS AND FOLLOW-UP CARE |
|
Right to decline provision of private health insurance: Under New York State law, hospitals must notify sexual assault patients, orally and in writing, of their right to decline to provide private health insurance information for billing for a forensic rape examination (FRE). If a sexual assault patient declines to provide such information, the hospital is prohibited from billing the patient or their insurance company for the FRE. Instead, the hospital may bill the OVS for the FRE. A minor patient may sign the FRE claim form so the facility can seek reimbursement for the sexual assault examination through the FRE program; however, it must be reasonable to conclude that the minor understands what they are signing and why.
Hospitals are required to advise sexual assault patients orally and in writing that they may decline to provide information about private health insurance benefits if they believe that provision of such information will substantially interfere with their privacy or safety. If patients so decline, then with the patient’s consent, OVS will be billed directly.
Follow-up PEP costs beyond the initial 7-day period and the costs of follow-up medical treatment needed as a result of the sexual assault will, for insured patients, continue to be reimbursed through the survivor/patient’s insurance, Medicaid, or another insurance program because OVS is the payor of last resort; however, OVS may consider the patient’s out-of-pocket responsibility for reimbursement. If a sexual assault patient is not insured or is a minor, a full OVS claim application should be filed. Minors are permitted to sign only the FRE claim form.
Follow-Up of the Exposed Individual
| RECOMMENDATIONS |
|
Abbreviations: PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis. |
Initial and Ongoing Follow-Up
Initial follow-up within 48 hours: Clinicians should follow up with the exposed individual within 48 hours, either by telephone call or in person, to assess PEP tolerability and adherence and to confirm access to the medications required to complete the full 28-day PEP regimen. If the patient has difficulty accessing the prescribed PEP medications, a social worker or patient navigator should be engaged to explore options and assist with medication access.
Follow-up care is necessary for patients taking PEP medications, to monitor for adverse effects and maximize adherence. Patients who report adverse effects by telephone should be evaluated in person if they require a physical examination (e.g., new rash or severe gastrointestinal symptoms such as abdominal pain, nausea, vomiting, and diarrhea). If the patient does not tolerate the recommended regimen well, an early switch to an alternative regimen is encouraged to improve adherence. Consultation with an experienced HIV care provider is advised when a patient’s PEP regimen must be changed.
Discuss the best method of contact for any adolescent or young adult who does not wish to disclose HIV exposure to parent(s) or guardian(s) and make sure to note the confidential phone number or method of contact.
Adherence support: Follow-up should also include discussions of daily adherence and reminders to complete the full 28 days of PEP. Clinicians should be aware of community resources for medical and supportive counseling/adherence services that a patient may need following non-occupational exposure.
Resources for PEP for providers and patients can be found at the NYSDOH website.
Ongoing follow-up: After the initial follow-up within 48 hours, a care provider or member of the PEP care team (such as a registered nurse, social worker, or patient navigator) should follow up with the patient by telephone or in-person visit by week 2 to further assess for adverse effects and confirm access to the medications required to complete the full 28-day course of PEP.
Patients who experience intolerable adverse effects may require in-person evaluation by a healthcare provider. Consultation with an experienced HIV care provider is advised if a switch to an alternative PEP regimen is required.
Care providers should pay particular attention to any symptoms suggestive of acute retroviral syndrome.
Risk Reduction
Transition to PrEP: Patients who remain at high risk of exposure after completing a course of non-occupational PEP and who are negative for HIV at the time of the 4-week HIV test should be offered PrEP, to begin immediately after the last dose of non-occupational PEP.
In a case-control study in Barcelona of possible predictors for HIV seroconversion among individuals using non-occupational PEP, independent factors associated with HIV seroconversion included being a man who has sex with men (MSM), having a known partner with HIV, taking a previous course of PEP, and having prior sexually transmitted infections (STIs) Leal(b), et al. 2016. Several observational cohort studies have noted high rates of HIV seroconversion among PEP users beyond the initial 3-month period after a potential exposure to HIV. These seroconversions are likely due to ongoing risk behaviors that may have been prevented by repeated courses of PEP or, more suitably, use of PrEP. At a large sexual health clinic in London where PEP was prescribed to 530 MSM over a 6-month period in 2013, 183 men received repeat PEP, and the incidence of repeat PEP was 24 per 100 person-years. Among the 57 men who acquired HIV, 12 could not be ruled out as experiencing PEP failure, and HIV incidence was 7.6 per 100 person-years Whitlock, et al. 2017. High rates of incident HIV have also been seen among non-occupational PEP recipients in Amsterdam, Australia, and Boston Poynten, et al. 2009; Heuker, et al. 2012; Jain, et al. 2015.
| Resources |
Follow-Up of Sexual Assault Patients
If a sexual assault patient is too distraught to engage in discussion and decision-making about PEP, then the care provider should encourage the individual to take a single dose of PEP and revisit the discussion the following day. The risk of taking one dose is minimal, and the efficacy that would be lost if delayed a whole day may be salvaged. If the individual decides to defer the decision to initiate PEP, then a follow-up visit within 24 hours should be scheduled to ensure that PEP is started as soon as possible and no later than 72 hours post exposure.
Resources and support for sexual assault patients: Sexual assault patients may require additional resources and support to ensure adherence to the daily PEP regimen and completion of the 28-day course. In a retrospective cohort study in Nairobi, Kenya, PEP was initiated in only 54% of cases involving sexual assault, and victims had low overall rates of completion of PEP (34%) and low rates (10%) of repeat HIV testing at 3 months Muriuki, et al. 2017. Similar low rates of PEP completion (27%) were noted in sexual assault patients at an academic medical center in Boston, MA Krause, et al. 2014.
Specific factors in this population may influence the acceptance of PEP. For instance, an analysis of forensic nurse examinations in the Mid-Atlantic region of the United States found that patients with injuries to the anus or genitalia were more likely to initiate PEP than patients with injuries to the face or head Draughon Moret, et al. 2016. These data suggest that sexual assault patients may need additional in-person visits or follow-up telephone calls from patient navigators, and social workers, and medical monitoring for adverse effects.
The treating clinician, preferably a sexual assault forensic examiner (SAFE), must coordinate care to encourage medical follow-up and adherence to PEP. The rape crisis advocate may become the crucial link between the sexual assault patient and the care provider, clarifying communication and facilitating follow-up care for the patient. When the patient does not have a primary care provider or has difficulty arranging access to a clinician experienced in HIV PEP, this link is especially important. Support from the advocate increases the likelihood that the sexual assault patient will adhere to the PEP regimen and that the primary care provider, PEP prescriber, or SAFE will be notified of medical problems. The advocate can also ensure that problems are addressed expeditiously as they arise.
| KEY POINT |
|
| SELECTED GOOD PRACTICE REMINDERS |
Follow-Up of the Exposed Individual
Follow-Up for Non-Occupational Exposures
Follow-Up for Sexual Assault Exposures
|
Sequential HIV Testing and Laboratory Monitoring
| RECOMMENDATIONS |
All ExposuresHIV Testing at 4 and 12 Weeks Post Exposure
Sequential HIV Testing and Laboratory Monitoring: If Acute HIV Is Suspected
Routine Laboratory Testing
Serial HIV Testing in Children
|
Abbreviations: Ab, antibody; Ag, antigen; FDA, U.S. Food and Drug Administration; PEP, post-exposure prophylaxis; POC, point-of-care. |
During the 28-day PEP treatment period, laboratory tests may be indicated to monitor for adverse effects of treatment. The timing and specific testing indicated varies based on the PEP regimen used (see Table 6, below).
Renal and liver function tests may be repeated during the 28-day follow-up period in the event of abnormal baseline renal or liver function tests (grade 1 abnormalities or higher). In one New York City PEP cohort, only 32 individuals (2.9%) and 95 individuals (8.5%) had abnormal renal function or liver function tests at baseline Mikati, et al. 2019. Follow-up testing found mostly grade 1 abnormalities, and no PEP regimens were changed because of renal function or liver function abnormalities. Repeat renal and liver function testing is advised for patients with decreased urine output, abdominal pain, nausea, vomiting, jaundice, or diarrhea.
Repeat sexually transmitted infection (STI) screening for non-occupational PEP following sexual exposure should also be considered at week 2 to assess for possible bacterial STI infection at the time of the potential HIV exposure, which would not have been detected with baseline testing. Screening should include chlamydia, gonorrhea, syphilis, and trichomoniasis if symptoms are present.
Sequential HIV testing (beyond the baseline): If HIV is transmitted during an exposure, seroconversion will generally occur within 2 to 4 weeks Cardo, et al. 1997; Ciesielski and Metler 1997; Joyce, et al. 2015. HIV testing at baseline, 4 weeks, and 12 weeks is recommended for all individuals who experience a high-risk exposure, even if PEP is declined.
Recommended HIV test: Point-of-care HIV tests in general are slightly less sensitive than laboratory-based HIV tests; therefore, exposed individuals should be tested with laboratory-based HIV tests whenever possible. An HIV-1/2 Ag/Ab combination immunoassay is the recommended serologic screening test. Point-of-care HIV tests that are Ab/Ab combination immunoassays are acceptable for follow-up testing.
HIV testing at 6 months after exposure is no longer recommended: Late seroconversion (i.e., after 3 months) is rare Ciesielski and Metler 1997; Ridzon, et al. 1997 but has occurred after completion of PEP Terzi, et al. 2007. It is unclear whether these rare events were related to the original or subsequent exposures. This committee believes that because of the infrequency of late seroconversion and the increased sensitivity of standard HIV tests to detect early infection and seroconversion, the benefit of routinely testing all exposed individuals for HIV at 6 months after exposure is outweighed by the added anxiety and significant consequences of an additional 3 months of precautions and testing for exposed individuals.
Laboratory monitoring: Table 6, below, includes recommended laboratory monitoring for patients who initiate a 28-day course of PEP. Serial HIV testing is recommended even if a patient declines PEP.
| Abbreviations: anti-HBs, hepatitis B surface antibody; CBC, complete blood count; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PEP, post-exposure prophylaxis; RPR, rapid plasma reagin. | ||
| Table 6: Recommended Monitoring After PEP Initiation | ||
| Monitoring Test or Activity | Frequency | Notes |
| Clinic visit |
|
Follow-ups at 48 hours and 2 weeks may be conducted by telephone call. |
| HIV-1/2 antigen/antibody combination immunoassay (recommended even if the exposed individual declines PEP) |
|
HIV specialist consultation: Immediate consultation with a clinician experienced in managing antiretroviral therapy is advised to determine optimal treatment options if the exposed individual’s sequential test confirms HIV infection. |
| Serum liver enzymes, blood urea nitrogen, creatinine, CBC |
|
|
| Pregnancy test |
|
Only if exposed individual is of childbearing capacity. |
| HBsAg, anti-HBs |
|
Patients with a reactive anti-HBs test result need not repeat an HBsAg test. |
| HCV antibody |
|
If source patient has known HCV viremia or unknown status, HCV antibody testing should be performed at baseline as well as 24 weeks after an initial nonreactive test result. |
| HCV RNA |
|
If source patient has known HCV viremia or unknown status, HCV RNA should be performed during HIV testing at weeks 4 and 12. |
| RPR, 3-site screening for gonorrhea and chlamydia |
|
|
Download Table 6: Recommended Monitoring After PEP Initiation Printable PDF
Management of Potential Exposure to Hepatitis B Virus
| RECOMMENDATIONS |
|
Abbreviations: anti-HBs, hepatitis B surface antibody; HBIG, hepatitis B immune globulin; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus. Notes:
|
Risk of HBV transmission: The risk of HBV transmission from an occupational exposure is significantly greater than the risk of HIV transmission and ranges from 1% to 31% depending on the presence of hepatitis B e antigen (HBeAg), which is a marker of active replication Schillie, et al. 2013.
Average risk of HBV transmission after needlestick (compared with HIV) CDC(a) 2001; Schillie, et al. 2013:
- HBV: 1.0% to 31.0%
- HBeAg+: 22% to 31%
- HBeAg-: 1.0% to 6.0%
- HIV: 0.3%
Factors that may increase the risk of sexual transmission include degree of viremia in the source, sex with multiple partners, history of sexually transmitted infections (including HIV), or any disruption of mucous membranes.
Any area exposed to blood or bodily fluid, including via needlestick, should be washed with soap and water as soon as possible after exposure. No data are available to suggest that the use of bleach or other antiseptic agents reduces transmission Schillie, et al. 2013.
HBV vaccine: When considering PEP for HBV exposure, evaluation of both the source’s HBsAg status and the exposed individual’s vaccination status is necessary (see below). Even if the risk of exposure to HBV is not deemed significant, HBV vaccination is advised for all non-HBV-immune individuals. Household, sex, and needle sharing contacts of HBsAg-positive individuals should be identified and vaccinated according to the guidelines for patients exposed to known HBsAg-positive individuals, and the source should be referred for evaluation and treatment of HBV infection.
Both the first dose of the HBV vaccine and, if indicated, HBIG should be administered as soon as possible after HBV exposure. The HBV vaccine should be administered within 24 hours post exposure, and HBIG should be administered within 7 days (ideally) and not later than 14 days post exposure.
- The 3-dose vaccine (e.g., Recombivax-HB, Engerix-B) is administered at 0, 1 to 2, and 6 months.
- The 2-dose vaccine (e.g., Heplisav-B) is administered at day 0 and 1 month later.
- Hepatitis A vaccination can be combined with hepatitis B (e.g., Twinrix) in a 3-dose series.
Anti-HBs should be obtained within 1 to 2 months after completion of the last dose of the vaccine.
See the Centers for Disease Control and Prevention (CDC) Vaccine Recommendations on Hepatitis B and American Academy of Pediatrics Care of the Adolescent After an Acute Sexual Assault Crawford-Jakubiak, et al. 2017.
Initiation of the HBV vaccine series within 12 to 24 hours post exposure has been demonstrated to be 70% to 90% effective in preventing HBV infection Schillie, et al. 2013. The combination of vaccine and HBIG achieves a similar level of efficacy Redeker, et al. 1975; Perrillo, et al. 1984. Among known nonresponders to vaccination, one dose of HBIG is 70% to 90% effective in preventing HBV when administered within 7 days of percutaneous HBV exposure Weinbaum, et al. 2003, Beasley, et al. 1983. The maximum effective interval for prophylaxis is likely within 14 days for sexual exposure Redeker, et al. 1975; Szmuness, et al. 1980; Perrillo, et al. 1984; Roumeliotou-Karayannis, et al. 1986; Papaevangelou, et al. 1987. It should be noted that a brief period of HBsAg positivity, reflecting a false-positive value, can be seen after vaccination Rysgaard, et al. 2012.
Pregnant women can safely receive both the HBV 3-dose vaccine series and HBIG. However, to date, there are no data available on the use of the newer 2-dose vaccine in pregnant patients, children, or patients on hemodialysis. Both the standard 3-dose vaccine and immunoglobulin are thought to be safe for both adult and pediatric patients; the 2-dose vaccine is not approved for patients younger than 18 years CDC(a) 2001; FDA 2023. Adverse effects of the vaccines, also present at the same rate in placebo, include pain at the injection site and fever CDC(a) 2001. HBIG is also safe for administration; there is no history of transmission of viral hepatitis or HIV through HBIG because the viruses are screened, inactivated, and eliminated during production of HBIG. Although anaphylactic reactions to HBIG or other immunoglobulin preparations are rare, if a patient does have a history of anaphylaxis after receipt of immunoglobulin, HBIG should not be given.
| KEY POINT |
|
Table 7, below, shows indicated treatment for individuals exposed to HBV, based on the status of the source. For the most current information regarding HBV post-exposure management, please refer to the CDC Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices.
| Abbreviations: anti-HBs, hepatitis B surface antibody; HBIG, hepatitis B immune globulin; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IM, intramuscular; PEP, post-exposure prophylaxis.
Notes:
|
|||
| Table 7: Recommended PEP for Hepatitis B Virus [a] | |||
| Exposed Individual Vaccination Status | Source is HBsAg-Positive | Source is HBsAg Negative or Not Available | Source is Not Available; Known High-Risk [b] |
| Indicated treatment for EXPOSED individual: | |||
| Unvaccinated/non-immune |
|
Initiate HBV vaccine series. |
Treat as if source is HBsAg-positive. |
| Previously vaccinated with completed HBV series; known responder [c] | No treatment. | ||
| Previously vaccinated with completed HBV series; known nonresponder [c] |
|
No treatment. | Treat as if source is HBsAg-positive. |
| Previously vaccinated with completed HBV series; unknown antibody response |
|
No treatment. | Treat as if source is HBsAg-positive. |
| Undergoing vaccination at time of exposure |
|
Complete vaccine series. | |
Management of Potential Exposure to Hepatitis C Virus
| RECOMMENDATIONS |
|
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; PEP, post-exposure prophylaxis. |
For more information on HCV, see the following NYSDOH AI guidelines:
- Hepatitis C Screening, Testing, and Diagnosis in Adults
- Pretreatment Assessment in Adults With Chronic Hepatitis C Infection
- Treatment of Chronic Hepatitis C Infection in Adults
Risk of HCV transmission: The risk of transmission of HCV is significantly greater than the risk of HIV transmission after bloodborne exposure. In cases of occupational exposure, the risk of HCV infection following a needlestick is 1.8%, whereas the risk of HIV infection is 0.3% Beltrami, et al. 2000. The risk of HCV transmission from a single mucous membrane exposure is negligible, except when the potential exposure is through receptive anal intercourse.
Factors that may increase the risk of sexual transmission include sex with multiple partners, history of sexually transmitted infections (including HIV), or any other practice that might disrupt mucous membranes (e.g., fisting or use of sex toys).
The following activities carry risk of HCV transmission:
- Blood-to-blood contact, including through sharing of personal care items, such as razors or toothbrushes, that may have been exposed to another individual’s blood; occupational needlestick injuries; and sharing needles, syringes, intranasal straws, or other equipment to inject or inhale drugs
- Sexual activity, particularly anal receptive intercourse
- Receipt of blood, plasma, organs, tissue, or semen
- Perinatal transmission
HCV is not spread via food or water and is not transmitted by:
- Sharing of eating utensils
- Hugging, kissing, or holding hands
- Coughing or sneezing
- Breastfeeding: HCV is not transmitted by breastfeeding; however, HCV is spread by infected blood. Therefore, if the HCV-positive mother’s nipples and/or surrounding areola are cracked and bleeding, she should stop nursing temporarily.
- Clinicians should advise women who may have been exposed to HIV to avoid breastfeeding for 3 months after the exposure (see guideline section Selecting and Initiating a 28-Day PEP Regimen > PEP During Pregnancy or Breastfeeding).
HCV testing of source: If the source is tested for HCV antibody and found to be positive, follow-up testing is necessary to confirm the source’s status. HCV RNA may be used as the confirmatory test. If the source tests positive with an HCV RNA test, the exposed individual should be managed as if the source has chronic HCV. If the source patient has recent risks for new HCV acquisition or the risk is unknown, consider nucleic acid amplification testing (NAAT) for HCV RNA as an initial test.
Figure 5: Evaluation of HCV Exposure Risk and Recommended Follow-Up
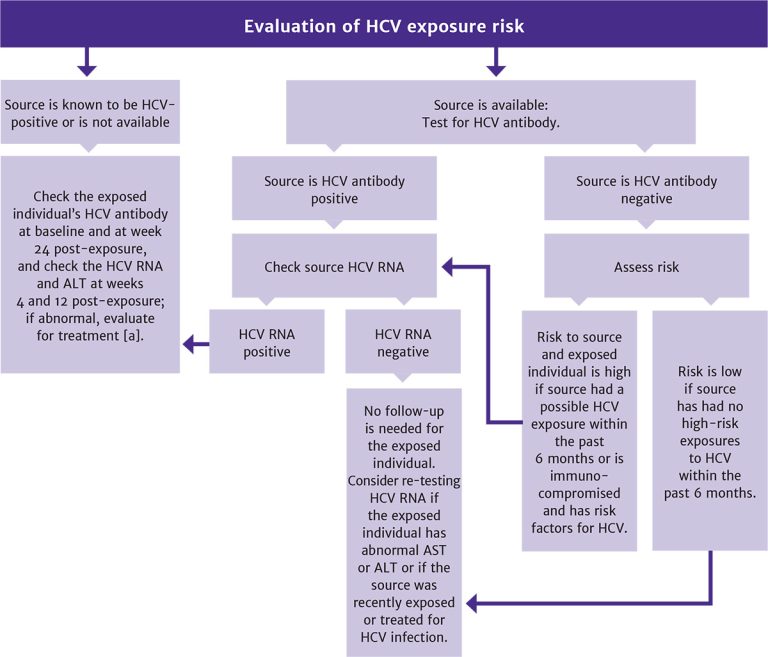
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus.
Note:
- If at any time the serum ALT level is elevated, repeat HCV RNA testing to evaluate for acute HCV infection. If HCV infection is identified, refer to a clinician with experience in treating HCV for medical management (see NYSDOH AI guideline Treatment of Chronic Hepatitis C Virus Infection in Adults).
Download figure: Evaluation of HCV Exposure Risk and Recommended Follow-Up
PEP for HCV: Currently, research has identified no effective prophylaxis for HCV infection. Immunoglobulin and antiviral agents are not recommended for HCV PEP. However, if an individual becomes acutely infected with HCV and is diagnosed at that time, immediate referral to a clinician experienced in the treatment of HCV is strongly recommended. Currently, the best regimen or duration of therapy for acute HCV is unknown, even with the availability of direct-acting HCV antiviral therapy. Patients should be managed according to genotype, liver disease progression, and history of previous HCV treatment, if any.
Observation for a period of 8 to 12 weeks post infection is reasonable to assess for possible spontaneous resolution of acute HCV Ghany, et al. 2009, and clinical trials are underway to assess the value of treatment with direct-acting antivirals for acute HCV infection. Whether treatment with direct-acting antiviral agents is appropriate will depend upon the individual scenario Boerekamps, et al. 2019; Chromy, et al. 2019; Naggie, et al. 2019.
Follow-up: For individuals who are exposed to a source with HCV, regular follow-up with HCV RNA testing is recommended in addition to HCV antibody testing. HCV RNA testing can identify acute infection within 2 weeks of exposure, whereas the antibody test may not provide an accurate result for up to several months after acute infection (i.e., during the “window period”). Seroconversion with the enzyme-linked immunosorbent assay (ELISA) antibody test occurs in 50% of patients who are infected within 9 weeks of exposure, in 80% of patients within 15 weeks of exposure, and in at least 97% of patients within 6 months of exposure CDC(a) 2001. The ELISA test is highly sensitive but relatively nonspecific, resulting in a low positive predictive value in low-prevalence populations. Positive HCV ELISA antibody test results require confirmation by a quantitative viral load assay, such as an HCV polymerase chain reaction assay. This committee recommends linking newly diagnosed patients to HCV care for monitoring and assessment for treatment.
| KEY POINT |
|
All Recommendations
| ALL RECOMMENDATIONS: PEP TO PREVENT HIV INFECTION |
First Dose of PEP and Management of the Exposure Site
Exposure Risk EvaluationAll Exposures
Sexual Assault Exposure
|
Abbreviations: Ab, antibody; Ag, antigen; ALT, alanine aminotransferase; anti-HBs, hepatitis B surface antibody; ART, antiretroviral therapy; ARV, antiretroviral; CrCl, creatinine clearance; FDA, U.S. Food and Drug Administration; HBIG, hepatitis B immune globulin; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; INSTI, integrase strand transfer inhibitor; PEP, post-exposure prophylaxis; PI, protease inhibitor; POC, point-of-care; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection. Notes:
|
All Good Practices
| ALL GOOD PRACTICES: PEP TO PREVENT HIV INFECTION |
First Dose of PEP and Management of the Exposure Site
Exposure Risk Evaluation
Source HIV Status and Management
Baseline Testing of the Exposed Individual
Baseline Testing Following Sexual Assault Exposures
Selecting and Initiating a 28-Day Course of PEP
Counseling and Patient Education
Follow-Up of the Exposed Individual
Follow-Up for Non-Occupational Exposures
Follow-Up for Sexual Assault Exposures
|
All Tables, Figures, and Boxes
Tables
- Table 1: Baseline Testing Based on Age of Exposed Individual and Type of Exposure
- Table 2: Preferred PEP Regimen for Patients Who Weigh ≥40 kg
- Table 3: Alternative PEP Regimens for Patients Who Weigh ≥40 kg
- Table 4: PEP Regimens for Patients 2 to 12 Years Old Who Weigh <40 kg
- Table 5: Antiretroviral Medications to Avoid for Post-Exposure Prophylaxis
- Table 6: Recommended Monitoring After PEP Initiation
- Table 7: Recommended PEP for Hepatitis B Virus
Figures
- Figure 1: Sequence of Events Following HIV Exposure, With and Without Administration of PEP
- Figure 2: Occupational HIV Exposure: PEP and Exposure Management When Reported Within 72 Hours
- Figure 3: Non-Occupational HIV Exposure: PEP and Exposure Management When Reported Within 72 Hours
- Figure 4: Sexual Assault HIV Exposure: Post-Exposure Prophylaxis and Exposure Management When Reported Within 72 Hours
- Figure 5: Evaluation of Hepatitis C Virus Exposure Risk and Recommended Follow-Up
Boxes
- Box 1: Risk per 10,000 Exposures of Acquiring HIV From an Infected Source, and Factors That Increase Risk
- Box 2: Risk of HIV Transmission From a Source With HIV
- Box 3: Non-Occupational Exposure Evaluation: Risks and Indications for Post-Exposure Prophylaxis
- Box 4: Source HIV Testing
- Box 5: Clinician-to-Clinician Communication
- Box 6: HIV Testing When the Source of an Occupational Exposure Is Unable to Consent
- Box 7: PEP Patient Education Checklist
Supplementary Materials
Employer Responsibilities in PEP Management to Prevent HIV Infection Following an Occupational Exposure
NYSDOH AIDS Institute, June 2020
Requirements: Organizations that employ health professionals or others who are at risk for occupational exposure to blood, body fluids, or other potentially infectious materials are generally required to establish policies and procedures that guide the management of such exposures.
Employers must conform to the OSHA Bloodborne Pathogens Standard (OSHA Bloodborne Pathogens Standard 29 CFR § 1910.1030 and Compliance Directive CPL 02-02-069, 11/27/01, Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens), which are applicable to New York public employers under the New York Public Employee Safety and Health (PESH) Act (Labor Law § 27-a) and regulations (12 NYCRR Part 800). OSHA and PESH standards regarding occupational exposure to bloodborne pathogens are identical. These regulations require that a management plan is in place.
Employee access to post-exposure services: The employer should ensure that any employee who sustains an occupational exposure has access to post-exposure services within 1 to 2 hours of a reported event. Services must be available 24 hours per day, 7 days per week. Organizations that do not have onsite occupational health services are encouraged to form agreements or contracts with another facility, Emergency Department, or private practitioner for such services.
Definition of individuals covered: New York State regulations apply to staff, employees, or volunteers in the performance of employment or professional duties who work in:
- A medical or dental office
- A facility regulated, authorized, or supervised by the Department of Health, Office of Mental Health, Office of Mental Retardation and Developmental Disabilities, Office of Children and Family Services, Office of Alcoholism and Substance Abuse Services, or the Department of Correctional Services
- Emergency response employee (paid or volunteer, including an emergency medical technician, a firefighter, a law enforcement officer or local correctional officer, or medical staff)
Post-exposure policies should define who is included as an “employee” for purposes of providing care. In addition to staff who are employed by an organization (e.g., nurses, laboratory personnel, housekeepers), consideration must be given to whether other individuals (e.g., medical/nursing students, house staff, attending physicians, volunteers, and pre-hospital care personnel) will be covered by the institution’s policy. In addition, the scope of services that will be provided must be delineated (e.g., laboratory testing, occupational health services, prophylactic drugs or vaccines), including whether there are limitations within the categories of individuals covered, particularly regarding workers’ compensation benefits.
Access to services: Exposed workers who sustain an occupational exposure should be ensured access to post-exposure services within 1 or 2 hours of a reported event. This may require 24-hour and weekend coverage. Procedures should identify how workers access services during regular work hours and, if different, how they access services during evening, night, or weekend shifts. Organizations that do not have onsite occupational health services should consider forming agreements or contracts with another facility or private practitioner for such services.
Post-exposure services for exposures to all bloodborne pathogens include but are not limited to:
- Post-exposure evaluation and follow-up post-exposure vaccinations
- Arrangements for a full course of PEP medications, at no cost to the employee
- Care provided under the supervision of a licensed physician or other licensed healthcare professional
- Availability of a rapid HIV test for source testing
- Supportive counseling
Federal law requires covered employers to ensure that all medical evaluations and procedures, vaccines, and post-exposure prophylaxis are made available to the employee within a reasonable time and at a reasonable location and are made available at no cost to the employee (OSHA, 29 CFR, Part 1910.2030, CPL 2-02.069, 11/27/01, Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens).
PESH and OSHA’s Bloodborne Pathogens Standards indicate that the covered employer is responsible for all costs associated with an exposure incident. An employer may not require any out-of-pocket expenditures on behalf of the employee, such as requiring the employee to utilize workers’ compensation if prepayment is required or compelling an employee to use health insurance to cover these expenses unless the employer pays all premiums and deductible costs associated with the employees’ health insurance. In addition to services listed above, the NYSDOH AI guideline PEP to Prevent HIV Infection states that the following should be considered by the employer when establishing plans for providing PEP for HIV exposure:
- Who will perform the post-exposure evaluation.
- Who will provide counseling to the exposed worker regarding the exposure and indications for PEP (for off-hour exposures as well).
- How PEP will be made available within 2 hours of an exposure.
- How a 7-day supply of PEP will be made available for urgent use.
- Who will be given authority for releasing drugs for this purpose.
- How the exposed worker will obtain PEP medications to complete the 28-day regimen.
Determining the HIV status of the exposure source: Procedures to facilitate rapid evaluation and voluntary testing for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and other bloodborne pathogens and disclosure of related information of the source individual should be in place.
The employer is responsible for establishing and implementing policies to protect the confidentiality of both the exposed employee and the exposure source (New York Public Health Law §§ 2135, 2782; 10 NYCRR § 63.6).
Access to source HIV-related information: New York law and regulations (Public Health Law § 2781(6)(e); 10 NYCRR § 63.8(m)) authorize disclosure of existing HIV-related information to healthcare providers of those who have been exposed in the workplace when significant risk exposure has occurred.
When the source is already known to be infected with HBV, HCV, or HIV, testing for the source individual’s known HBV, HCV, or HIV status does not need to be repeated. Testing for other bloodborne pathogens should still occur.
If the exposed worker is part of the healthcare team, he/she may have access to the medical record and know the HIV status of the source, as well as information about drug resistance. Information related to drug regimens, and, if available, resistance information should be made available to the exposed employee’s healthcare provider to determine the best regimen for the employee. However, initiation of PEP should not be delayed while awaiting this information.
HIV testing of the source: Consistent with recommendations by the Centers for Disease Control and Prevention (CDC), and the U.S. Department of Labor, OSHA mandates that medical facilities subject to OSHA authority use rapid HIV antibody tests when testing the source after potential exposure to a bloodborne pathogen. The CDC recommends testing with an HIV-1/2 antibody/antigen combination immunoassay.
- The source should be tested as soon as possible to determine HIV infectivity.
- Results of the source individual’s HIV testing should be made available to the exposed worker’s healthcare provider. Patient authorization for the release of this information is not required for necessary communication of information between care providers for timely treatment of the exposed worker.
Source has the capacity to consent for HIV testing: Informed consent from the source should be obtained. If consent is not obtained for HIV testing, the employer should document that consent cannot be obtained and testing cannot be performed (see Box 6: HIV Testing When the Source of an Occupational Exposure Is Unable to Consent).
Source does not have the capacity to consent for HIV testing: If the source is comatose or is determined by his or her attending professional to lack the mental capacity to consent, and the source is not expected to recover in time for the exposed individual to receive appropriate medical treatment, the Health Care Proxy Law and Family Health Care Decisions Act (FHCDA) give healthcare providers the ability to locate someone who has the legal authority to consent to HIV testing (the healthcare agent or FHCDA Surrogate).
New York regulations [§§ 63.3(d)(7), 63.8(n)] also authorize anonymous testing when no individual authorized to consent on behalf of the source is immediately available.
An anonymous test* may be performed if: The healthcare agent or FHCDA Surrogate, who has the legal authority to consent, is not available or reasonably likely to become available in time for the exposed individual to receive appropriate medical treatment and the exposed individual will benefit medically by knowing the source’s HIV test results or the source is deceased.
*The law requires that results of anonymous source testing are given only to the healthcare provider of the exposed individual solely for assisting the exposed individual in making appropriate decisions regarding post-exposure medical treatment. The results of the test cannot be disclosed to the source or placed in the source’s medical record. The source may be told that the exposure occurred and that an HIV test was performed. The source should be offered confidential testing so that they may have access to information about his/her own HIV status.
Worker’s compensation program: The Workers’ Compensation Law has specific implications for employees exposed to HIV, as well as those rare cases that result in seroconversion. Individuals who manage such exposures should be familiar with these implications because they should be able to counsel employees and refer them for legal and medical assistance accordingly. The organization’s workers’ compensation provider should be contacted as situations arise.
NYS Worker’s Compensation Board:
- Website: http://www.wcb.ny.gov/
- Worker benefits and information regarding how to file a claim: http://www.wcb.ny.gov/content/main/Workers/Workers.jsp
- Advocate for Injured Workers, for questions related to injured workers:
(877) 632-4996
(800) 580-6665
Email: advinjwkr@wcb.ny.gov
Preventing transmission of bloodborne pathogens: As part of the employer’s plan to prevent transmission of bloodborne pathogens, the following measures can be taken to avoid injuries:
- Elimination of unnecessary use of needles or other sharps
- Use of devices with safety features
- Verification of training and compliance with safety features
- Avoidance of needle recapping
- Planning before beginning any procedure using needles or other sharps for safe handling and prompt disposal in sharps disposal containers
- Promotion of education and safe work practices for handling needles and other sharps
For more information about prevention of needlestick injuries, refer to the National Institute for Occupational Safety and Health Alert: Preventing Needlestick Injuries in Health Care Settings.
Even when effective prevention measures are implemented, exposures to blood and bodily fluid still occur. Employers of personnel covered by the OSHA Bloodborne Pathogens Standard are obligated to provide post-exposure care, including prophylaxis, at no cost to the employee. The employer may subsequently attempt to obtain reimbursement from workers’ compensation.
Documentation: Information that should be recorded after an occupational exposure to HIV has occurred includes the following, which the clinician should record in the exposed worker’s confidential medical record:
- Date and time of the exposure
- Details of the procedure being performed and the use of protective equipment at the time of the exposure
- Type, severity, and amount of fluid to which the worker was exposed
- Details about the source individual
- Whether HIV testing of the source was performed
- Medical documentation that provides details about post-exposure management
- If the occupationally exposed individual declines PEP, the clinician should document this decision in the individual’s medical record.
Specific OSHA requirements regarding documentation may be found at Safety and Health Topics: Bloodborne Pathogens and Needlestick Prevention.
Services for Sexual Assault Patients
NYSDOH AIDS Institute, June 2020
New York State (NYS) Public Health Law 2805-i requires that hospitals providing treatment to survivors of sexual assault advise the patient of the availability of services provided by the local rape crisis or victim assistance organization and secure such services as requested by the patient.
Role of the rape crisis advocate: The primary role of the rape crisis advocate is to provide the patient with emotional support, advocacy, information, counseling, and accompaniment services, and to facilitate informed decision-making at a time when the patient may be in crisis. Advocates do not provide healthcare or collect evidence; however, they can enhance the efforts of healthcare staff through the provision of information regarding medical and legal options. For information about rape crisis services, see NYSDOH Sexual Violence Prevention Program. The NYSDOH, with other State agencies, healthcare facilities, and professional organizations, provides technical assistance on sexual assault issues.
Sexual Assault Forensic Examiner (SAFE): The initial response that a survivor of rape or sexual assault receives when seeking healthcare or reporting the crime has a profound influence on that individual’s subsequent recovery. Engagement of healthcare practitioners from the SAFE program helps improve the care that survivors of sexual assault receive. The NYSDOH certifies all appropriately qualified individuals as SAFEs. A SAFE is a specially trained registered nurse, nurse practitioner, physician, or physician’s assistant.
NYS public health law requires that the NYSDOH establish standards for and certify SAFE hospital programs. All SAFE Designated Hospitals have a SAFE available either on site or on-call within 60 minutes of the sexual assault patient’s arrival at the hospital, except under exigent circumstances (NYS Public Health Law 2805-i). In NYS, the standard of care for survivors of rape and sexual assault presenting at healthcare settings includes comprehensive high-quality medical care, collection of forensic evidence, and respectful and sensitive treatment. The NYSDOH recommends the use of SAFEs in all hospitals to assist in meeting this standard. The SAFE should be an active participant in the discussion regarding initiation of HIV post-exposure prophylaxis (PEP). SAFEs help to ensure the best medical, legal, and psychological outcomes for the adult survivor of sexual assault and provide compassionate emotional support. They are trained to provide care to survivors of sexual assault and to collect and preserve forensic evidence to support prosecution if the patient decides to report the crime to law enforcement.
| RESOURCES |
Reimbursement for SAFE services: Provider reimbursement under the Office of Victim Services (OVS) FRE Direct Reimbursement Program is intended to cover the forensic examiner’s services, including pharmaceuticals related to a sexual assault forensic examination. This reimbursement includes the cost of the initial 7-day starter pack of PEP if the care provider determines a risk of HIV exposure. Claim forms for reimbursement under the Direct Reimbursement Program can be found in each Sexual Offense Evidence Collection Kit and be downloaded from the OVS website.
Documentation of a visit to a facility that provides a forensic medical examination satisfies the OVS reporting requirement, thereby providing survivors who are either unwilling or unable to report the crime to the police the opportunity to file a regular compensation claim. Survivors of sexual assault may also contact a Rape Crisis Center or Victim Advocate Program in their county or region for assistance in filing regular compensation claims with OVS, particularly when an emergency award is needed from the OVS (see below). Many of these agencies have 24-hour hotlines. For more information and a list of Victim Advocate Programs and other resources, consult the OVS website.
The OVS has an “emergency award” procedure in addition to its normal compensation process to ensure continued availability of PEP for survivors of sexual assault beyond the initial 7-day starter pack supply. Advocates who know the community connections and procedures to expedite the process should work with the exposed individual. The process for requesting an emergency award is as follows: 1) Claimant files a regular claim application with the OVS, indicating that medication for HIV PEP is necessary, and requests an emergency award. 2) OVS makes an expedited determination for the purposes of the emergency award. 3) If the OVS determines it can grant an emergency award, up to $2,500, then OVS directly reimburses pharmacy providers on behalf of the claimant.
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
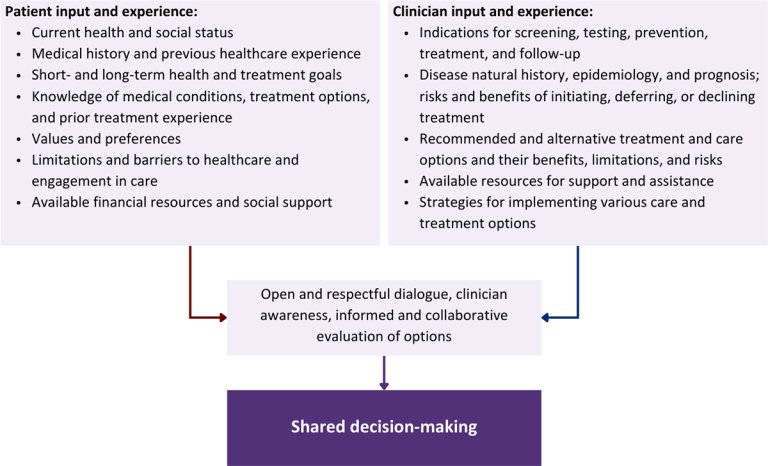
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Advisory Committee for HIV and STD Prevention. HIV prevention through early detection and treatment of other sexually transmitted diseases--United States. Recommendations of the Advisory Committee for HIV and STD Prevention. MMWR Recomm Rep 1998;47(RR-12):1-24. [PMID: 9701544]
Al-Hajjar S. H., Frayha H. H., Al-Hazmi M., et al. Prevention of HIV-1 transmission with postexposure prophylaxis after inadvertent infected blood transfusion. AIDS 2014;28(10):1539-41. [PMID: 24896805]
Albert J., Wahlberg J., Leitner T., et al. Analysis of a rape case by direct sequencing of the human immunodeficiency virus type 1 pol and gag genes. J Virol 1994;68(9):5918-24. [PMID: 7520096]
American Academy of Pediatrics. 1997 Report on the committee on infectious diseases: hepatitis B; 1997. https://www.google.com/books/edition/1997_Report_of_the_Committee_on_Infectio/oraAQgAACAAJ
American Academy of Pediatrics. Issues related to human immunodeficiency virus transmission in schools, child care, medical settings, the home, and community. American Academy of Pediatrics. Committee of Pediatric AIDS and Committee on Infectious Diseases. Pediatrics 1999;104(2 Pt 1):318-24. [PMID: 10429018]
American Academy of Pediatrics. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics 2013;131(2):391-96. [PMID: 23359577]
Andreo S. M., Barra L. A., Costa L. J., et al. HIV type 1 transmission by human bite. AIDS Res Hum Retroviruses 2004;20(4):349-50. [PMID: 15157352]
Attia S., Egger M., Muller M., et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009;23(11):1397-1404. [PMID: 19381076]
Auvert B., Taljaard D., Lagarde E., et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 trial. PLoS Med 2005;2(11):e298. [PMID: 16231970]
Bader M. S., McKinsey D. S. Postexposure prophylaxis for common infectious diseases. Am Fam Physician 2013;88(1):25-32. [PMID: 23939603]
Baeten J., Donnell D., Ndase P., et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367(5):399-410. [PMID: 22784037]
Bailey R. C., Moses S., Parker C. B., et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007;369(9562):643-56. [PMID: 17321310]
Bartholomew C. F., Jones A. M. Human bites: a rare risk factor for HIV transmission. AIDS 2006;20(4):631-32. [PMID: 16470132]
Beasley R. P., Hwang L. Y., Stevens C. E., et al. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology 1983;3(2):135-41. [PMID: 6339349]
Beltrami E. M., Cheingsong R., Heneine W. M., et al. Antiretroviral drug resistance in human immunodeficiency virus-infected source patients for occupational exposures to healthcare workers. Infect Control Hosp Epidemiol 2003;24(10):724-30. [PMID: 14587931]
Beltrami E. M., Williams I. T., Shapiro C. N., et al. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev 2000;13(3):385-407. [PMID: 10885983]
Beymer M. R., Weiss R. E., Bolan R. K., et al. Differentiating nonoccupational postexposure prophylaxis seroconverters and non-seroconverters in a community-based clinic in Los Angeles, California. Open Forum Infect Dis 2017;4(2):ofx061. [PMID: 28596981]
Black R. J. Animal studies of prophylaxis. Am J Med 1997;102(5b):39-44. [PMID: 9845495]
Boerekamps A., De Weggheleire A., van den Berk G. E., et al. Treatment of acute hepatitis C genotypes 1 and 4 with 8 weeks of grazoprevir plus elbasvir (DAHHS2): an open-label, multicentre, single-arm, phase 3b trial. Lancet Gastroenterol Hepatol 2019;4(4):269-77. [PMID: 30660617]
Cardo D. M., Culver D. H., Ciesielski C. A., et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med 1997;337(21):1485-90. [PMID: 9366579]
Casey E. A., Querna K., Masters N. T., et al. Patterns of intimate partner violence and sexual risk behavior among young heterosexually active men. J Sex Res 2016;53(2):239-50. [PMID: 26158212]
CDC. Transmission of HIV possibly associated with exposure of mucous membrane to contaminated blood. MMWR Morb Mortal Wkly Rep 1997;46(27):620-23. [PMID: 9218647]
CDC. Announcement: updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV - United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65(17):458. [PMID: 27149423]
CDC. Sexually transmitted diseases (STDs): treatment and screening. 2017 Apr 19. https://www.cdc.gov/std/treatment/ [accessed 2017 Jun 30]
CDC. Sexually transmitted diseases (STDs): sexually transmitted disease surveillance 2017. 2018 Jul 24. https://www.cdc.gov/std/stats17/toc.htm [accessed 2019 Mar 11]
CDC. Violence prevention: intimate partner violence: fast facts: preventing teen dating violence. 2023 Jan 27. https://www.cdc.gov/violenceprevention/intimatepartnerviolence/teendatingviolence/fastfact.html [accessed 2019 Jul 3]
CDC(a). Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001;50(RR-5):1-43. [PMID: 11349873]
CDC(a). HIV risk behaviors. 2019 Nov 13. https://www.cdc.gov/hiv/risk/estimates/riskbehaviors.html [accessed 2018 Feb 22]
CDC(b). Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000. MMWR Morb Mortal Wkly Rep 2001;49(51-52):1153-56. [PMID: 11198946]
CDC(b). Sexually transmitted disease surveillance 2018. 2019 Oct 8. https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf [accessed 2020 May 24]
Chromy D., Mandorfer M., Bucsics T., et al. High efficacy of interferon-free therapy for acute hepatitis C in HIV-positive patients. United European Gastroenterol J 2019;7(4):507-16. [PMID: 31065368]
Ciesielski C. A., Metler R. P. Duration of time between exposure and seroconversion in healthcare workers with occupationally acquired infection with human immunodeficiency virus. Am J Med 1997;102(5b):115-16. [PMID: 9845512]
Claydon E., Murphy S., Osborne E. M., et al. Rape and HIV. Int J STD AIDS 1991;2(3):200-201. [PMID: 1863649]
Cohen M. S., Chen Y. Q., McCauley M., et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375(9):830-39. [PMID: 27424812]
Cohen M. S., Chen Y. Q., McCauley M., et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493-505. [PMID: 21767103]
Cottrell M. L., Garrett K. L., Prince H. M. A., et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J Antimicrob Chemother 2017;72(6):1731-40. [PMID: 28369415]
Crawford-Jakubiak J. E., Alderman E. M., Leventhal J. M. Care of the adolescent after an acute sexual assault. Pediatrics 2017;139(3):e20164243. [PMID: 28242861]
Cresswell F. V., Ellis J., Hartley J., et al. A systematic review of risk of HIV transmission through biting or spitting: implications for policy. HIV Med 2018;19(8):532-40. [PMID: 29687590]
Day S., Mears A., Bond K., et al. Post-exposure HIV prophylaxis following sexual exposure: a retrospective audit against recent draft BASHH guidance. Sex Transm Infect 2006;82(3):236-37. [PMID: 16731676]
DeGruttola V., Seage G. R., Mayer K. H., et al. Infectiousness of HIV between male homosexual partners. J Clin Epidemiol 1989;42(9):849-56. [PMID: 2789269]
Delaney K. P., Hanson D. L., Masciotra S., et al. Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clin Infect Dis 2017;64(1):53-59. [PMID: 27737954]
DHHS. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. 2023 Jan 31. https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new-guidelines [accessed 2018 Feb 1]
Di Giovanni C., Berlin F., Casterella P., et al. Prevalence of HIV antibody among a group of paraphilic sex offenders. J Acquir Immune Defic Syndr 1991;4(6):633-37. [PMID: 2023104]
Dominguez K. L. Management of HIV-infected children in the home and institutional settings. Care of children and infections control in schools, day care, hospital settings, home, foster care, and adoption. Pediatr Clin North Am 2000;47(1):203-39. [PMID: 10697649]
Draughon Moret J. E., Hauda W. E., Price B., et al. Nonoccupational postexposure human immunodeficiency virus prophylaxis: acceptance following sexual assault. Nurs Res 2016;65(1):47-54. [PMID: 26657480]
European Study Group on Heterosexual Transmission of HIV. Comparison of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on Heterosexual Transmission of HIV. BMJ 1992;304(6830):809-13. [PMID: 1392708]
Fajman N., Wright R. Use of antiretroviral HIV post-exposure prophylaxis in sexually abused children and adolescents treated in an inner-city pediatric emergency department. Child Abuse Negl 2006;30(8):919-27. [PMID: 16939690]
Fätkenheuer G., Jessen H., Stoehr A., et al. PEPDar: a randomized prospective noninferiority study of ritonavir-boosted darunavir for HIV post-exposure prophylaxis. HIV Med 2016;17(6):453-59. [PMID: 27166295]
FDA. Isentress (raltegravir) film-coated or chewable tablets, for oral use. 2013 Aug. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022145s029lbl.pdf [accessed 2018 Aug 13]
FDA. Heplisav-B [hepatitis B vaccine (recombinant), adjuvanted] solution for intramuscular injection. 2023 May. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf [accessed 2019 Feb 28]
Ford N., Mayer K. H. World Health Organization guidelines on postexposure prophylaxis for HIV: recommendations for a public health approach. Clin Infect Dis 2015;60(Suppl 3):s161-64. [PMID: 25972497]
Ford N., Shubber Z., Calmy A., et al. Choice of antiretroviral drugs for postexposure prophylaxis for adults and adolescents: a systematic review. Clin Infect Dis 2015;60(Suppl 3):s170-76. [PMID: 25972499]
Foster R., McAllister J., Read T. R., et al. Single-tablet emtricitabine-rilpivirine-tenofovir as HIV postexposure prophylaxis in men who have sex with men. Clin Infect Dis 2015;61(8):1336-41. [PMID: 26123937]
Gellert G. A., Berkowitz C. D., Gellert M. J., et al. Testing the sexually abused child for the HIV antibody: issues for the social worker. Soc Work 1993;38(4):389-94. [PMID: 8362274]
Ghany M. G., Strader D. B., Thomas D. L., et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49(4):1335-74. [PMID: 19330875]
Golub S. A., Rosenthal L., Cohen D. E., et al. Determinants of high-risk sexual behavior during post-exposure prophylaxis to prevent HIV infection. AIDS Behav 2008;12(6):852-59. [PMID: 17682938]
Grant R. M., Lama J. R., Anderson P. L., et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363(27):2587-99. [PMID: 21091279]
Gray R. H., Kigozi G., Serwadda D., et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007;369(9562):657-66. [PMID: 17321311]
Grossin C., Sibille I., de la Grandmaison G. L., et al. Analysis of 418 cases of sexual assault. Forensic Sci Int 2003;131(2-3):125-30. [PMID: 12590050]
Günthard H. F., Saag M. S., Benson C. A., et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA 2016;316(2):191-210. [PMID: 27404187]
Hamlyn E., McAllister J., Winston A., et al. Is screening for sexually transmitted infections in men who have sex with men who receive non-occupational HIV post-exposure prophylaxis worthwhile?. Sex Transm Infect 2006;82(1):21-23. [PMID: 16461596]
Heuker J., Sonder G. J., Stolte I., et al. High HIV incidence among MSM prescribed postexposure prophylaxis, 2000-2009: indications for ongoing sexual risk behaviour. AIDS 2012;26(4):505-12. [PMID: 22156963]
Hudgens M. G., Longini I. M., Halloran M. E., et al. Estimating the transmission probability of human immunodeficiency virus in injecting drug users in Thailand. J Royal Stat Soc. Series C (Appl Stat) 2001;50(1):1-14. https://www.jstor.org/stable/2680837
Hudgens M. G., Longini I. M., Vanichseni S., et al. Subtype-specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am J Epidemiol 2002;155(2):159-68. [PMID: 11790680]
Irvine C., Egan K. J., Shubber Z., et al. Efficacy of HIV postexposure prophylaxis: systematic review and meta-analysis of nonhuman primate studies. Clin Infect Dis 2015;60(Suppl 3):s165-69. [PMID: 25972498]
Jain S., Oldenburg C. E., Mimiaga M. J., et al. Subsequent HIV infection among men who have sex with men who used non-occupational post-exposure prophylaxis at a Boston community health center: 1997-2013. AIDS Patient Care STDS 2015;29(1):20-25. [PMID: 25369451]
Jamani S., Gulholm T., Poynten I. M., et al. Timing and frequency of chlamydia and gonorrhoea testing in a cross-sectional study of HIV postexposure prophylaxis recipients. Sex Transm Infect 2013;89(7):604-6. [PMID: 23698512]
James, S. E.. The report of the 2015 U.S. Transgender Survey. 2016 Dec. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf [accessed 2018 Apr 25]
Jin F., Jansson J., Law M., et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS 2010;24(6):907-13. [PMID: 20139750]
Johnson L. F., Lewis D. A. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis 2008;35(11):946-59. [PMID: 18685546]
Jones J. S., Rossman L., Diegel R., et al. Sexual assault in postmenopausal women: epidemiology and patterns of genital injury. Am J Emerg Med 2009;27(8):922-29. [PMID: 19857408]
Jones J. S., Rossman L., Hartman M., et al. Anogenital injuries in adolescents after consensual sexual intercourse. Acad Emerg Med 2003;10(12):1378-83. [PMID: 14644791]
Joyce M. P., Kuhar D., Brooks J. T. Notes from the field: occupationally acquired HIV infection among health care workers - United States, 1985-2013. MMWR Morb Mortal Wkly Rep 2015;63(53):1245-46. [PMID: 25577991]
Kahn J. O., Martin J. N., Roland M. E., et al. Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: the San Francisco PEP Study. J Infect Dis 2001;183(5):707-14. [PMID: 11181146]
Kaplan E. H., Heimer R. A model-based estimate of HIV infectivity via needle sharing. J Acquir Immune Defic Syndr 1992;5(11):1116-18. [PMID: 1403641]
Kleppa E., Holmen S. D., Lillebø K., et al. Cervical ectopy: associations with sexually transmitted infections and HIV. A cross-sectional study of high school students in rural South Africa. Sex Transm Infect 2015;91(2):124-29. [PMID: 25281761]
Klot J. F., Auerbach J. D., Berry M. R. Sexual violence and HIV transmission: summary proceedings of a scientific research planning meeting. Am J Reprod Immunol 2013;69 Suppl 1:5-19. [PMID: 23157400]
Krause K. H., Lewis-O'Connor A., Berger A., et al. Current practice of HIV postexposure prophylaxis treatment for sexual assault patients in an emergency department. Womens Health Issues 2014;24(4):e407-12. [PMID: 24981399]
Kuhar D. T., Henderson D. K., Struble K. A., et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013;34(9):875-92. [PMID: 23917901]
Kumar T., Sampsel K., Stiell I. G. Two, three, and four-drug regimens for HIV post-exposure prophylaxis in a North American sexual assault victim population. Am J Emerg Med 2017;35(12):1798-1803. [PMID: 28596030]
Laitinen F. A., Grundmann O., Ernst E. J. Factors that influence the variability in findings of anogenital injury in adolescent/adult sexual assault victims: a review of the forensic literature. Am J Forensic Med Pathol 2013;34(3):286-94. [PMID: 23835534]
Larsen M. L., Hilden M., Lidegaard Ø. Sexual assault: a descriptive study of 2500 female victims over a 10-year period. BJOG 2015;122(4):577-84. [PMID: 25315463]
Leal(a) L., León A., Torres B., et al. A randomized clinical trial comparing ritonavir-boosted lopinavir versus raltegravir each with tenofovir plus emtricitabine for post-exposure prophylaxis for HIV infection. J Antimicrob Chemother 2016;71(7):1987-93. [PMID: 26994089]
Leal(b) L., Torres B., León A., et al. Predictive factors for HIV seroconversion among individuals attending a specialized center after an HIV risk exposure: a case-control study. AIDS Res Hum Retroviruses 2016;32(10-11):1016-21. [PMID: 27457508]
LeGoff J., Weiss H. A., Gresenguet G., et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS 2007;21(12):1569-78. [PMID: 17630552]
Leynaert B., Downs A. M., de Vincenzi I. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol 1998;148(1):88-96. [PMID: 9663408]
Lindegren M. L., Hanson I. C., Hammett T. A., et al. Sexual abuse of children: intersection with the HIV epidemic. Pediatrics 1998;102(4):E46. [PMID: 9755283]
Lunding S., Katzenstein T. L., Kronborg G., et al. The Danish PEP Registry: experience with the use of postexposure prophylaxis (PEP) following sexual exposure to HIV from 1998 to 2006. Sex Transm Dis 2010;37(1):49-52. [PMID: 19734819]
Martinez de Tejada B., Gayet-Ageron A., Winterfeld U., et al. Birth defects after exposure to efavirenz-based antiretroviral therapy at conception/first trimester of pregnancy: a multicohort analysis. J Acquir Immune Defic Syndr 2019;80(3):316-24. [PMID: 30570524]
Mastro T. D., de Vincenzi I. Probabilities of sexual HIV-1 transmission. AIDS 1996;10 Suppl A:S75-82. [PMID: 8883613]
Mayer K. H., Jones D., Oldenburg C., et al. Optimal HIV postexposure prophylaxis regimen completion with single tablet daily elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine compared with more frequent dosing regimens. J Acquir Immune Defic Syndr 2017;75(5):535-39. [PMID: 28696345]
Mayer K. H., Mimiaga M. J., Cohen D., et al. Tenofovir DF plus lamivudine or emtricitabine for nonoccupational postexposure prophylaxis (NPEP) in a Boston community health center. J Acquir Immune Defic Syndr 2008;47(4):494-99. [PMID: 18176318]
Mayer K. H., Mimiaga M. J., Gelman M., et al. Raltegravir, tenofovir DF, and emtricitabine for postexposure prophylaxis to prevent the sexual transmission of HIV: safety, tolerability, and adherence. J Acquir Immune Defic Syndr 2012;59(4):354-59. [PMID: 22267017]
Mayer K. H., Venkatesh K. K. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol 2011;65(3):308-16. [PMID: 21214660]
McAllister J., Read P., McNulty A., et al. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014;15(1):13-22. [PMID: 24007390]
McAllister J., Towns J. M., McNulty A., et al. Dolutegravir with tenofovir disoproxil fumarate-emtricitabine as HIV postexposure prophylaxis in gay and bisexual men. AIDS 2017;31(9):1291-95. [PMID: 28301425]
McDougal S. J., Alexander J., Dhanireddy S., et al. Non-occupational post-exposure prophylaxis for HIV: 10-year retrospective analysis in Seattle, Washington. PLoS One 2014;9(8):e105030. [PMID: 25140868]
Medscape. Needle-stick guideline. 2021 Jul 1. https://emedicine.medscape.com/article/784812-overview [accessed 2018 Jun 14]
Mikati T., Crawley A., Daskalakis D. C. Are routine renal and liver labs testing among PEP patients on TDF/FTC/DTV necessary? Abstract 983. CROI; 2019 Mar 4-7. https://www.croiconference.org/sessions/are-routine-renal-and-liver-labs-testing-among-pep-patients-tdfftcdtv-necessary
Modjarrad K., Chamot E., Vermund S. H. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS 2008;22(16):2179-85. [PMID: 18832881]
Mulka L., Annandale D., Richardson C., et al. Raltegravir-based HIV postexposure prophylaxis (PEP) in a real-life clinical setting: fewer drug-drug interactions (DDIs) with improved adherence and tolerability. Sex Transm Infect 2016;92(2):107. [PMID: 26892929]
Muriuki E. M., Kimani J., Machuki Z., et al. Sexual assault and HIV postexposure prophylaxis at an urban African hospital. AIDS Patient Care STDS 2017;31(6):255-60. [PMID: 28605228]
Murphy S., Kitchen V., Harris J. R., et al. Rape and subsequent seroconversion to HIV. BMJ 1989;299(6701):718. [PMID: 2508885]
Myles J. E., Hirozawa A., Katz M. H., et al. Postexposure prophylaxis for HIV after sexual assault. JAMA 2000;284(12):1516-18. [PMID: 11000643]
Naggie S., Fierer D. S., Hughes M. D., et al. Ledipasvir/sofosbuvir for 8 weeks to treat acute hepatitis C virus infections in men with human immunodeficiency virus infections: sofosbuvir-containing regimens without interferon for treatment of acute HCV in HIV-1 infected individuals. Clin Infect Dis 2019;69(3):514-22. [PMID: 31220220]
NIOSH. Preventing needlestick injuries in health care settings. Publication no. 2000-108. 1999 Nov. https://www.cdc.gov/niosh/docs/2000-108/ [accessed 2017 Jul 5]
Nwaiwu C. A., Egro F. M., Smith S., et al. Seroconversion rate among health care workers exposed to HIV-contaminated body fluids: the University of Pittsburgh 13-year experience. Am J Infect Control 2017;45(8):896-900. [PMID: 28449921]
NYSDOH. Sexual assault forensic examiner (SAFE) program. 2023 Jun. https://www.health.ny.gov/professionals/safe/ [accessed 2020 Jun 12]
Ogata-Aoki H., Higashi-Kuwata N., Hattori S. I., et al. Raltegravir blocks the infectivity of red-fluorescent-protein (mCherry)-labeled HIV-1JR-FL in the setting of post-exposure prophylaxis in NOD/SCID/Jak3(-/-) mice transplanted with human PBMCs. Antiviral Res 2018;149:78-88. [PMID: 28893602]
Oldenburg C. E., Jain S., Mayer K. H., et al. Post-exposure prophylaxis use and recurrent exposure to HIV among men who have sex with men who use crystal methamphetamine. Drug Alcohol Depend 2015;146:75-80. [PMID: 25482500]
Otten R. A., Smith D. K., Adams D. R., et al. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J Virol 2000;74(20):9771-75. [PMID: 11000253]
Page-Shafer K., Shiboski C. H., Osmond D. H., et al. Risk of HIV infection attributable to oral sex among men who have sex with men and in the population of men who have sex with men. AIDS 2002;16(17):2350-52. [PMID: 12441814]
Papaevangelou G., Roumeliotou-Karayannis A., Richardson S. C., et al. Postexposure immunoprophylaxis of spouses of patients with acute viral hepatitis B. International Symposium on Viral Hepatitis and Liver Disease; 1987 May 26-28. https://doi.org/10.1002/jmv.1890210411
Papenburg J., Blais D., Moore D., et al. Pediatric injuries from needles discarded in the community: epidemiology and risk of seroconversion. Pediatrics 2008;122(2):e487-92. [PMID: 18676535]
Paquet A., Evans M. C., Petropoulos C., et al. Significant reductions in the prevalence of protease inhibitor and 3-class resistance: recent trends in a large HIV-1 protease/reverse transcriptase database. Abstract H2-800. 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2011 Sep 17-20. http://www.natap.org/2011/ICAAC/ICAAC_68.htm
Parkin J. M., Murphy M., Anderson J., et al. Tolerability and side-effects of post-exposure prophylaxis for HIV infection. Lancet 2000;355(9205):722-23. [PMID: 10703807]
Patel P., Borkowf C. B., Brooks J. T., et al. Estimating per-act HIV transmission risk: a systematic review. AIDS 2014;28(10):1509-19. [PMID: 24809629]
Patterson B. K., Landay A., Siegel J. N., et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol 2002;161(3):867-73. [PMID: 12213715]
Patterson K. B., Leone P. A., Fiscus S. A., et al. Frequent detection of acute HIV infection in pregnant women. AIDS 2007;21(17):2303-8. [PMID: 18090278]
Penazzato M., Dominguez K., Cotton M., et al. Choice of antiretroviral drugs for postexposure prophylaxis for children: a systematic review. Clin Infect Dis 2015;60 Suppl 3:S177-81. [PMID: 25972500]
Perrillo R. P., Campbell C. R., Strang S., et al. Immune globulin and hepatitis B immune globulin. Prophylactic measures for intimate contacts exposed to acute type B hepatitis. Arch Intern Med 1984;144(1):81-85. [PMID: 6362597]
Pilcher C. D., Tien H. C., Eron J. J., et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis 2004;189(10):1785-92. [PMID: 15122514]
Poynten I. M., Jin F., Mao L., et al. Nonoccupational postexposure prophylaxis, subsequent risk behaviour and HIV incidence in a cohort of Australian homosexual men. AIDS 2009;23(9):1119-26. [PMID: 19417578]
Pretty I. A., Anderson G. S., Sweet D. J. Human bites and the risk of human immunodeficiency virus transmission. Am J Forensic Med Pathol 1999;20(3):232-39. [PMID: 10507789]
PRN Notebook. Ulcerating STDs and HIV: a cause for concern. 2005 Jun. https://www.prn.org/index.php/coinfections/article/ulcerating_stds_and_hiv_79 [accessed 2020 Jun 1]
Quinn T. C., Wawer M. J., Sewankambo N., et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000;342(13):921-29. [PMID: 10738050]
Raj A., Santana M. C., La Marche A., et al. Perpetration of intimate partner violence associated with sexual risk behaviors among young adult men. Am J Public Health 2006;96(10):1873-78. [PMID: 16670216]
Redeker A. G., Mosley J. W., Gocke D. J., et al. Hepatitis B immune globulin as a prophylactic measure for spouses exposed to acute type B hepatitis. N Engl J Med 1975;293(21):1055-59. [PMID: 1101065]
Richman K. M., Rickman L. S. The potential for transmission of human immunodeficiency virus through human bites. J Acquir Immune Defic Syndr (1988) 1993;6(4):402-6. [PMID: 8455145]
Ridzon R., Gallagher K., Ciesielski C., et al. Simultaneous transmission of human immunodeficiency virus and hepatitis C virus from a needle-stick injury. N Engl J Med 1997;336(13):919-22. [PMID: 9070472]
Riggs N., Houry D., Long G., et al. Analysis of 1,076 cases of sexual assault. Ann Emerg Med 2000;35(4):358-62. [PMID: 10736122]
Rodger A. J., Cambiano V., Bruun T., et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019;393(10189):2428-38. [PMID: 31056293]
Rodger A. J., Cambiano V., Bruun T., et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316(2):171-81. [PMID: 27404185]
Roumeliotou-Karayannis A., Dandolos E., Richardson S. C., et al. Immunogenicity of a reduced dose of recombinant hepatitis B vaccine. Vaccine 1986;4(2):93-94. [PMID: 2941929]
Rysgaard C. D., Morris C. S., Drees D., et al. Positive hepatitis B surface antigen tests due to recent vaccination: a persistent problem. BMC Clin Pathol 2012;12:15. [PMID: 23006828]
Sachs C. J., Chu L. D. Predictors of genitorectal injury in female victims of suspected sexual assault. Acad Emerg Med 2002;9(2):146-51. [PMID: 11825841]
Schillie S., Murphy T. V., Sawyer M., et al. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013;62(RR-10):1-19. [PMID: 24352112]
Smith D. K., Grohskopf L. A., Black R. J., et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep 2005;54(RR-2):1-20. [PMID: 15660015]
Smith M. S., Foresman L., Lopez G. J., et al. Lasting effects of transient postinoculation tenofovir [9-R-(2-phosphonomethoxypropyl)adenine] treatment on SHIV(KU2) infection of rhesus macaques. Virology 2000;277(2):306-15. [PMID: 11080478]
Smith, S. G.. National Intimate Partner and Sexual Violence Survey: 2015 data brief – updated release. 2018 Nov. https://www.cdc.gov/violenceprevention/pdf/2015data-brief508.pdf [accessed 2019 Mar 11]
Sommers M. S., Brown K. M., Buschur C., et al. Injuries from intimate partner and sexual violence: significance and classification systems. J Forensic Leg Med 2012;19(5):250-63. [PMID: 22687765]
Spira A. I., Marx P. A., Patterson B. K., et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med 1996;183(1):215-25. [PMID: 8551225]
Stephenson R., Finneran C. Receipt and perpetration of intimate partner violence and condomless anal intercourse among gay and bisexual men in Atlanta. AIDS Behav 2017;21(8):2253-60. [PMID: 28176169]
Sugar N. F., Fine D. N., Eckert L. O. Physical injury after sexual assault: findings of a large case series. Am J Obstet Gynecol 2004;190(1):71-76. [PMID: 14749638]
Sultan B., Benn P., Waters L. Current perspectives in HIV post-exposure prophylaxis. HIV AIDS (Auckl) 2014;6:147-58. [PMID: 25368534]
Szmuness W., Stevens C. E., Harley E. J., et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med 1980;303(15):833-41. [PMID: 6997738]
Terzi R., Niero F., Iemoli E., et al. Late HIV seroconversion after non-occupational postexposure prophylaxis against HIV with concomitant hepatitis C virus seroconversion. AIDS 2007;21(2):262-63. [PMID: 17197828]
Thigpen M. C., Kebaabetswe P. M., Paxton L. A., et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367(5):423-34. [PMID: 22784038]
Tosini W., Muller P., Prazuck T., et al. Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation. AIDS 2010;24(15):2375-80. [PMID: 20729709]
Tovanabutra S., Robison V., Wongtrakul J., et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr 2002;29(3):275-83. [PMID: 11873077]
Tsai C. C., Emau P., Follis K. E., et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol 1998;72(5):4265-73. [PMID: 9557716]
Unger E. R., Fajman N. N., Maloney E. M., et al. Anogenital human papillomavirus in sexually abused and nonabused children: a multicenter study. Pediatrics 2011;128(3):e658-65. [PMID: 21844060]
Valin N., Fonquernie L., Daguenel A., et al. Evaluation of tolerability with the co-formulation elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate for post-HIV exposure prophylaxis. BMC Infect Dis 2016;16(1):718. [PMID: 27894270]
Van Rompay K. K., Berardi C. J., Aguirre N. L., et al. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS 1998;12(9):F79-83. [PMID: 9662190]
Van Rompay K. K., Miller M. D., Marthas M. L., et al. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J Virol 2000;74(4):1767-74. [PMID: 10644348]
Varghese B., Maher J. E., Peterman T. A., et al. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002;29(1):38-43. [PMID: 11773877]
Vidmar L., Poljak M., Tomazic J., et al. Transmission of HIV-1 by human bite. Lancet 1996;347(9017):1762. [PMID: 8656918]
Wall K. M., Kilembe W., Vwalika B., et al. Risk of heterosexual HIV transmission attributable to sexually transmitted infections and non-specific genital inflammation in Zambian discordant couples, 1994-2012. Int J Epidemiol 2017;46(5):1593-1606. [PMID: 28402442]
Wawer M. J., Gray R. H., Sewankambo N. K., et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005;191(9):1403-9. [PMID: 15809897]
Weinbaum C., Lyerla R., Margolis H. S. Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. MMWR Recomm Rep 2003;52(RR-1):1-36. [PMID: 12562146]
Weinbaum C., Williams I., Mast E. E., et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 2008;57(RR-8):1-20. [PMID: 18802412]
Weller S., Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev 2002;(1):CD003255. [PMID: 11869658]
Wertz J., Cesario J., Sackrison J., et al. Acute HIV infection in pregnancy: the case for third trimester rescreening. Case Rep Infect Dis 2011;2011:340817. [PMID: 22567467]
Whitlock G., McCormack C., Fearnley J., et al. High HIV incidence in men who have sex with men attending for postexposure prophylaxis: a service evaluation. Sex Transm Infect 2017;93(3):214-16. [PMID: 27412954]
Zamora A. B., Rivera M. O., Garcia-Algar O., et al. Detection of infectious human immunodeficiency type 1 virus in discarded syringes of intravenous drug users. Pediatr Infect Dis J 1998;17(7):655-57. [PMID: 9686738]
Zash R., Holmes L. B., Diseko M., et al. Update on neural tube defects with antiretroviral exposure in the Tsepamo Study, Botswana. AIDS; 2022 Jul 29-Aug 2. https://www.natap.org/2022/IAC/IAC_31.htm
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | June 25, 2020 |
| Date of current publication | April 17, 2023 |
| Highlights of changes, additions, and updates in the April 17, 2023 edition |
In Table 6: Recommended Monitoring After PEP Initiation, a note for RPR and 3-site screening for gonorrhea and chlamydia has been changed from “Consider repeat screening at week 2 for sexual exposures” to “Repeat screening at week 4 for sexual exposures.” |
| Intended users | New York State clinicians who provide post-exposure prophylaxis for individuals who report a potential exposure to HIV with a potential concomitant exposure to hepatitis B virus or hepatitis C virus |
| Lead author |
Elliott DeHaan, MD |
| Contributing author(s) |
Christine A. Kerr, MD; Aracelis Fernandez, MD; Lisa-Gaye Robinson, MD; Ruby Fayorsey, MD |
| Writing group |
Steven M. Fine, MD, PhD; Rona Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD |
| Author and writing group conflict of interest disclosures | There are no author or writing group conflict of interest disclosures. |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines |
NYSDOH AI Guidance |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on May 30, 2024

