Purpose of This Guidance
Date of current publication: September 26, 2023
Lead authors: Judith Griffin, MD; Sara Lorenz Taki, MD; Timothy J. Wiegand, MD
Writing group: Susan D. Whitley, MD; Sharon L. Stancliff, MD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Substance Use Guidelines Committee
Date of original publication: July 3, 2023
The New York State Department of Health AIDS Institute (NYSDOH AI) developed this guidance for primary care and other clinicians with patients who use stimulants to:
- Inform clinicians about different types of stimulants and current terminology for describing stimulants and stimulant use.
- Provide strategies for talking with patients about stimulant use and the associated risks, including opioid overdose.
- Summarize the treatment options for stimulant use disorder.
The guidance focuses on nonprescription stimulant substances, including cocaine and crack; methamphetamine, 3,4-methylenedioxy-methamphetamine (MDMA; hallucinogen with stimulant effects); and synthetic cathinones (bath salts).
Rising use and mortality: The results of the U.S. 2021 National Survey on Drug Use and Health indicate that among people aged ≥12 years, 4.8 million had used cocaine, 2.5 million had used methamphetamines, 2.2 million had used MDMA, and 107,000 had used synthetic stimulants (including cathinones) in the previous year SAMHSA 2023. In addition to overdose, misuse or chronic use of stimulants can cause or worsen psychosis, anger, paranoia, and cardiac and gastrointestinal problems NIDA 2023; Duflou 2020; Paulus and Stewart 2020; Reddy, et al. 2020; Kevil, et al. 2019; McKetin, et al. 2019; McKetin, et al. 2013; Darke, et al. 2008; Zweben, et al. 2004. Stimulant injection has been associated with an increased incidence of HIV and hepatitis C virus Cepeda, et al. 2020; Farrell, et al. 2019.
In the United States, drug overdose deaths involving methamphetamine increased from 547 in 1999 to 23,837 in 2020, and drug overdose deaths involving cocaine increased from 5,419 in 2014 to 19,447 in 2020 NIDA 2023, leading some to characterize the rising cocaine- and methamphetamine-related mortality as a fourth wave of the U.S. overdose crisis Ciccarone and Shoptaw 2022; Fischer, et al. 2021. In 2021 in the United States, approximately 66% of overdose deaths were attributed to concomitant use of stimulants and opioids: fentanyl with cocaine (16.6% of overdose deaths), fentanyl with methamphetamine (10.7%), and other opioids with stimulants (39.9%) CDC 2023. Much of the recent increase in mortality is attributed to concomitant use of fentanyl and stimulants and possibly to use of illicitly manufactured stimulants that contain fentanyl, although the extent of adulteration is unclear. In New York State, overdose deaths involving cocaine increased from 388 in 2010 to 1,320 in 2019, and 65% of the deaths in 2019 involved synthetic opioids NYSDOH 2023. Similarly, in New York City, fentanyl was present in 81% of cocaine overdoses and 66% of amphetamine-involved overdoses in 2020 NYC Health 2021.
| KEY POINTS |
|
Commonly Used Stimulants
“Stimulants” is the general term used to describe the many synthetic or naturally occurring substances that elevate mood and increase alertness, attention, and energy. These substances increase catecholamine levels and agonist activity at adrenergic receptors, which increases the release of dopamine and norepinephrine. Table 1, below, summarizes the types and characteristics of commonly used stimulants.
| Abbreviations: ADHD, attention-deficit hyperactivity disorder; GI, gastrointestinal; HBV, hepatitis B virus; HCV, hepatitis C virus; MDMA, 3,4-methylenedioxy-methamphetamine; SAMHSA, Substance Abuse and Mental Health Services Administration.
Notes:
|
|
| Table 1: Characteristics of Commonly Used Stimulants in Nonpregnant Adults [a] | |
| Stimulant Type [b] | Characteristics [c,d] |
| Cathinone, Synthetic | |
|
|
| Cocaine | |
|
|
| MDMA | |
|
|
| Methamphetamine | |
|
|
| Prescribed: Amphetamines and Amphetamine Derivatives | |
|
|
Download Table 1: Characteristics of Commonly Used Stimulants in Nonpregnant Adults Printable PDF
| RESOURCES |
|
Screening, Assessment, and Counseling
Screening and Assessment
The NYSDOH AI evidence-based guideline Substance Use Screening and Risk Assessment in Adults recommends annual substance use screening for all adult patients. A positive screening result for stimulant use or a history of stimulant use disorder or overdose should prompt an assessment of the patient’s level of risk. Stimulant use disorder is defined as the continued use of amphetamine-type substances, cocaine, or other stimulants leading to clinically significant impairment or distress; diagnosis is based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR) criteria. See the tables listed below in the NYSDOH AI guideline Substance Use Screening and Risk Assessment in Adults:
- Table 1: Recommended Validated Tools for Use in Medical Settings to Screen for Alcohol and Drug Use in Adults
- Table 2: Brief, Validated Risk Assessment Tools for Use in Medical Settings with Adults ≥ 18 Years Old
- Table 3: DSM-5 Diagnostic Criteria for Diagnosing and Classifying Substance Use Disorders
| KEY POINTS |
|
Patients may be concerned that substance use disclosure will bias their clinicians and have a negative effect on their care, or they may have previous experience with healthcare bias and legal ramifications. Before asking screening questions, assure patients that the detailed questions are intended to help clinicians offer appropriate treatment and services, and remind patients that responses are voluntary. Advise patients that answers will be documented in the medical record and available to the healthcare team. Medical records will not be released without the patient’s signed consent, except when subpoenaed by a court.
Box 1, below, describes an approach to talking with patients about substance use during the screening and assessment processes and ongoing conversations that may help to build trust in the clinician-patient relationship.
| Box 1: Communicating With Patients About Stimulant and Other Substance Use [a] |
|
|
Note:
|
Counseling and Medical Care for Patients Who Use Stimulants
Based on the clinical expertise of the author and the Substance Use Guidelines Committee, the strategies below can help clinicians engage patients in discussion of their stimulant use, harm reduction, and recommended medical care.
Effects of stimulant use: Ask patients who use stimulants about how the substance affects them negatively and positively and any harmful effects or symptoms that may be related. Understanding patients’ drug use experience can inform harm reduction and treatment goals. Clinicians can simply ask, “How do you think meth use is affecting you?”
Explore the effect of stimulant use on underlying medical and mental health conditions or symptoms in an objective, nonjudgmental manner. For example, stimulant use may cause or worsen cardiovascular conditions Reddy, et al. 2020; Kevil, et al. 2019; Darke, et al. 2017, and asking patients about symptoms associated with stimulant use (e.g., chest pain, shortness of breath, or headache) may help clinicians engage patients in a conversation focused on harm reduction or prevention. Examples include “Stimulants can raise your blood pressure” and “Have you experienced any shortness of breath?”
Stimulant use may worsen preexisting anxiety and depression and may cause or worsen psychotic symptoms including mania, paranoia, and delusions McKetin, et al. 2019; McKetin, et al. 2013; Darke, et al. 2008; Zweben, et al. 2004. Clinicians can state, “I am concerned that the meth use may be increasing your anxiety,” or ask, “Other people using ICE sometimes experience paranoia/delusions…have you experienced that?”
Do not assume symptoms are solely due to substance use and do not forego any clinical evaluation or treatment as routinely indicated. It is important to provide appropriate medical care for other conditions and symptoms in patients with ongoing stimulant use (e.g., initiating or continuing treatment for hypertension even if the patient continues to use stimulants).
The term “overamping” may be used by patients to describe the negative effects of overusing stimulants. Symptoms associated with overamping vary between individuals and include anxiety, paranoia, psychosis, seizure, palpitations, hypertension, hyperthermia, and cardiac and cerebrovascular events Ciccarone and Shoptaw 2022; Harding, et al. 2022. Overamping may not be an acute or discrete event, and different patients may define and experience it differently. Treatment for overamping includes symptom management (e.g., hypertension) and supportive care. Patients should be advised to hydrate, replenish electrolytes, and remain in a calm environment.
Withdrawal: Stimulant withdrawal symptoms may include fatigue, irritability, insomnia, poor concentration, anxiety, depression, and decreased ability to perform daily activities Ciccarone and Shoptaw 2022. Although stimulant withdrawal is not itself life-threatening, symptoms may persist for days or weeks in individuals who have a history of chronic use. Of note, there is also an increased risk of self-harm and suicide due to symptoms of depression and dysphoria related to stimulant withdrawal SAMHSA 2021; Lerner and Klein 2019.
Stimulant use with sex: Asking patients if and how they use stimulants before or during sex can inform harm reduction counseling. Sexualized stimulant use among men who have sex with men (MSM) is associated with engaging in behaviors that increase the risk of acquiring and transmitting HIV and other sexually transmitted and bloodborne infections (e.g., condomless sex, sex with multiple partners, and injection drug use) Strong, et al. 2022; Curtis, et al. 2020; Guerra, et al. 2020; Tomkins, et al. 2019. “Chemsex” or “party and play” is one type of sexualized drug use and involves the use of specific psychoactive drugs—commonly methamphetamines, γ-hydroxybutyrate (GHB), γ-butyrolactone (GBL), and mephedrone—by MSM to enhance sex Strong, et al. 2022. In general, chemsex is an event that involves casual or anonymous partners or group sex, lasts for an extended time, and is often arranged using digital apps Harm Reduction International 2021. “Slam sex” refers to the injection of methamphetamines or other stimulants during sex Schreck, et al. 2021.
For patient education resources, see ChemSex Harm Reduction: Safer Chemsex & Safer Sex Guides and Tweaker: Crystal Meth.
For information about HIV prevention see NYSDOH AI guidelines PEP to Prevent HIV Infection and PrEP to Prevent HIV and Promote Sexual Health; for information about STI prevention and treatment, see CDC STI Treatment Guidelines, 2021.
Harm reduction: In counseling patients, clinicians should take a nonjudgmental and supportive approach about the potential risks of stimulant use, including overdose, and strategies to reduce the risks. Patients who use stimulants, including those with occasional or episodic stimulant use, may underestimate their opioid overdose risk; all patients who use stimulants should be engaged in harm reduction counseling and encouraged to make a plan to prevent overdose.
Overdose prevention strategies: Counsel patients to:
- Assume all illicitly manufactured opioids will contain fentanyl or other high-potency synthetic opioids, and stimulants and counterfeit pills may contain these agents.
- When possible, test drugs with fentanyl and xylazine test strips or other drug-checking systems. Online sources include MATTERS (for New York State residents and programs, no charge), DanceSafe, and BTNX. Some NYS Authorized Syringe Exchange Sites may provide fentanyl test strips and other drug-checking systems.
- Avoid using drugs alone. Arrange for someone to check in; use phone- and web-based apps (e.g., Never Use Alone Inc. at 800-484-3731).
- When using any drug, start with a small amount.
- Carry naloxone (NLX), learn how to use it to reverse an opioid overdose, and encourage friends and contacts to do the same. The 4 mg NLX nasal spray formulation is available at pharmacies, at NYSDOH-Registered Opioid Overdose Prevention Programs (no charge), and through online resources including MATTERS (for NYS programs and residents, no charge) and NEXT Distro. NLX is covered by NYS Medicaid and the majority of private insurers.
See NYSDOH AI guideline Substance Use Harm Reduction in Medical Care > Box 1: Harm Reduction Resources in New York State (September 2023).
Treatment
Stimulant use disorder: If a patient is diagnosed with a stimulant use disorder, assess their readiness to change behavior, and advise them on the types of behavioral and pharmacologic treatment approaches that have been studied. Collaboration on treatment options best suited to the individual’s life and preferences is essential. It can be helpful to discuss barriers to care (e.g., housing, transportation, competing priorities) and positive supports and experiences that may facilitate treatment success. Develop a plan in collaboration with the patient using the principles of shared decision-making. Consider planning a feasible goal (e.g., reduced level of use, safer use) and schedule a follow-up appointment to reassess progress. A patient’s treatment goals may change over time and should be reassessed periodically.
Goals of treatment: Abstinence from stimulants may not be achievable for many individuals in the short term, and alternative treatment goals can lead to substantial improvements in the health and lives of those who use stimulants. With a harm reduction approach, treatment goals may include staying in care; treating co-occurring medical conditions; reducing use; reducing risky behaviors; improving mental health, and improving quality of life and other social indicators, such as employment, stable housing, and reduced risk of incarceration.
Treatment options: Evidence supports motivational interviewing, contingency management (CM), a community reinforcement approach, and cognitive behavioral therapy for the treatment of stimulant use disorder SAMHSA 2021. A prescription digital therapeutic app for stimulant and other substance use disorders (ReSET) has been approved by the U.S. Food and Drug Administration (FDA) as part of combination treatment Maricich, et al. 2022; FDA(a) 2017. These products are software applications for smartphones designed to deliver cognitive therapy and CM.
Systematic reviews and meta-analyses indicate that CM is more effective than other treatments for cocaine use disorder Bentzley, et al. 2021 and more effective than cognitive behavioral therapy, 12-step groups, and other behavioral strategies for stimulant use disorder De Crescenzo, et al. 2018. The goal of CM is to increase desired behavior (i.e., reducing substance use) by providing immediate reinforcing incentives, such as cash or vouchers when the target behavior occurs. However, providing a CM intervention in a real-world setting can be difficult SAMHSA 2021. In 2022, the Centers for Medicare and Medicaid Services (CMS) approved a CM pilot program for Medicaid enrollees in California.
There is not sufficient evidence on which to base general pharmacologic treatment recommendations, and no medications are approved by the FDA for the treatment of stimulant use disorder. In randomized, placebo-controlled clinical trials, several treatment regimens have been associated with reductions in stimulant use in some individuals with cocaine or methamphetamine use disorder (see Table 2, below). However, treatment response rates are generally low, and results are complicated by multiple factors, including low adherence rates high dropout rates, comorbid substance use disorders, and the classic use of abstinence as a primary outcome Brandt, et al. 2021.
| Abbreviations: NCBI, National Center for Biotechnology Information; XR, extended-release.
Notes:
|
|
| Table 2: Pharmacologic Treatment of Stimulant Use Disorder: Selected Randomized Controlled Clinical Trials [a] | |
| Study Medication(s) | Study Publication/NCBI Abstract or Full-Text Link |
| Amphetamine or Methamphetamine Use Disorder | |
| Mirtazapine |
|
| Injectable XR naltrexone plus XR oral bupropion |
|
| Cocaine Use Disorder | |
| Topiramate |
|
| XR oral mixed amphetamine salts [b] and topiramate |
|
| Sustained-release dextroamphetamine [b] |
|
| Disulfiram |
|
| KEY POINT |
|
Concomitant OUD treatment: Continue medication for opioid use disorder (OUD) in patients who also use stimulants, and counsel patients that OUD medications do not treat stimulant use. Stimulant use or use disorder has been associated with lower rates of OUD treatment initiation and retention in care Frost, et al. 2021. If a stimulant use disorder is adversely affecting OUD treatment, have a nonjudgmental discussion with the patient, reassess their treatment and harm reduction goals and strategies, counsel them to continue OUD treatment, and consider treatment for stimulant use disorder.
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
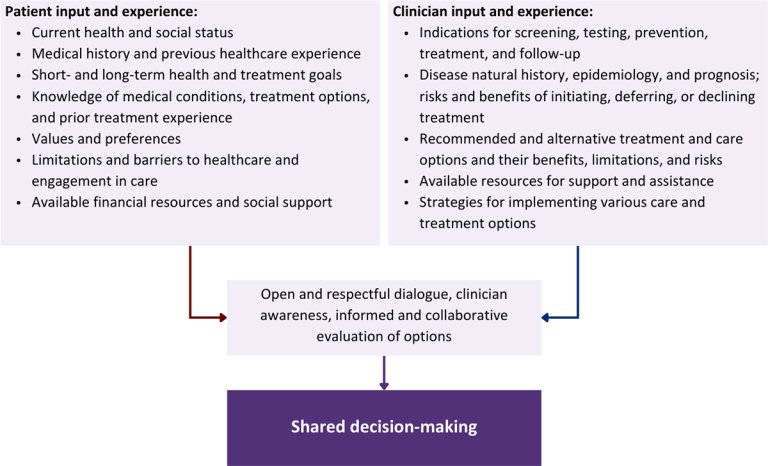
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Aldridge R. W., Story A., Hwang S. W., et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet 2018;391(10117):241-50. [PMID: 29137869]
Arum C., Fraser H., Artenie A. A., et al. Homelessness, unstable housing, and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Public Health 2021;6(5):e309-23. [PMID: 33780656]
Baldaçara L., Cogo-Moreira H., Parreira B. L., et al. Efficacy of topiramate in the treatment of crack cocaine dependence: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 2016;77(3):398-406. [PMID: 27046312]
Bartholow L. A., Huffman R. T. The necessity of a trauma-informed paradigm in substance use disorder services. J Am Psychiatr Nurses Assoc 2021;10783903211036496. [PMID: 34334012]
Bentzley B. S., Han S. S., Neuner S., et al. Comparison of treatments for cocaine use disorder among adults: a systematic review and meta-analysis. JAMA Netw Open 2021;4(5):e218049. [PMID: 33961037]
Brandt L., Chao T., Comer S. D., et al. Pharmacotherapeutic strategies for treating cocaine use disorder-what do we have to offer?. Addiction 2021;116(4):694-710. [PMID: 32888245]
British Columbia Centre on Substance Use. Stimulant use disorder: practice update. 2022 Jun. https://www.bccsu.ca/wp-content/uploads/2022/06/Stimulant-Use-Disorder-Practice-Update_June2022.pdf [accessed 2023 May 15]
Cano M., Oh S., Salas-Wright C. P., et al. Cocaine use and overdose mortality in the United States: evidence from two national data sources, 2002-2018. Drug Alcohol Depend 2020;214:108148. [PMID: 32702620]
CDC. SUDORS dashboard: fatal overdose data. 2023 Aug 25. https://www.cdc.gov/drugoverdose/fatal/dashboard/index.html [accessed 2023 May 1]
Cepeda J. A., Vickerman P., Bruneau J., et al. Estimating the contribution of stimulant injection to HIV and HCV epidemics among people who inject drugs and implications for harm reduction: a modeling analysis. Drug Alcohol Depend 2020;213:108135. [PMID: 32603976]
Ciccarone D., Shoptaw S. Understanding stimulant use and use disorders in a new era. Med Clin North Am 2022;106(1):81-97. [PMID: 34823736]
Coffin P. O., Santos G. M., Hern J., et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry 2020;77(3):246-55. [PMID: 31825466]
Colfax G. N., Santos G. M., Das M., et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry 2011;68(11):1168-75. [PMID: 22065532]
Curtis T. J., Rodger A. J., Burns F., et al. Patterns of sexualised recreational drug use and its association with risk behaviours and sexual health outcomes in men who have sex with men in London, UK: a comparison of cross-sectional studies conducted in 2013 and 2016. Sex Transm Infect 2020;96(3):197-203. [PMID: 31744928]
Darke S., Duflou J., Kaye S. Prevalence and nature of cardiovascular disease in methamphetamine-related death: a national study. Drug Alcohol Depend 2017;179:174-79. [PMID: 28787694]
Darke S., Kaye S., McKetin R., et al. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 2008;27(3):253-62. [PMID: 18368606]
De Crescenzo F., Ciabattini M., D'Alò G. L., et al. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med 2018;15(12):e1002715. [PMID: 30586362]
Duflou J. Psychostimulant use disorder and the heart. Addiction 2020;115(1):175-83. [PMID: 31321853]
Farrell M., Martin N. K., Stockings E., et al. Responding to global stimulant use: challenges and opportunities. Lancet 2019;394(10209):1652-67. [PMID: 31668409]
FDA(a). FDA permits marketing of mobile medical application for substance use disorder. 2017 Sep 14. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-mobile-medical-application-substance-use-disorder [accessed 2023 Jun 28]
FDA(b). Wellbutrin XL (bupropion hydrochloride extended-release) tablets for oral use. 2017 May. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021515s036lbl.pdf [accessed 2023 May 5]
Fischer B., O'Keefe-Markman C., Lee A. M., et al. 'Resurgent', 'twin' or 'silent' epidemic? A select data overview and observations on increasing psycho-stimulant use and harms in North America. Subst Abuse Treat Prev Policy 2021;16(1):17. [PMID: 33588896]
Frost M. C., Lampert H., Tsui J. I., et al. The impact of methamphetamine/amphetamine use on receipt and outcomes of medications for opioid use disorder: a systematic review. Addict Sci Clin Pract 2021;16(1):62. [PMID: 34635170]
Goulian A., Jauffret-Roustide M., Dambélé S., et al. A cultural and political difference: comparing the racial and social framing of population crack cocaine use between the United States and France. Harm Reduct J 2022;19(1):44. [PMID: 35550157]
Guerra F. M., Salway T. J., Beckett R., et al. Review of sexualized drug use associated with sexually transmitted and blood-borne infections in gay, bisexual and other men who have sex with men. Drug Alcohol Depend 2020;216:108237. [PMID: 33091811]
Harding R. W., Wagner K. T., Fiuty P., et al. "It's called overamping": experiences of overdose among people who use methamphetamine. Harm Reduct J 2022;19(1):4. [PMID: 35034643]
Harm Reduction International. Chemsex and harm reduction for gay men and other men who have sex with men. 2021 Jul. https://hri.global/wp-content/uploads/2022/10/HRI_Briefing_Chemsex_July_2021_Final-1.pdf [accessed 2023 Jun 27]
Johnson B. A., Ait-Daoud N., Wang X. Q., et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry 2013;70(12):1338-46. [PMID: 24132249]
Karsberg S., Hesse M., Pedersen M. M., et al. The impact of poly-traumatization on treatment outcomes in young people with substance use disorders. BMC Psychiatry 2021;21(1):140. [PMID: 33685430]
Kevil C. G., Goeders N. E., Woolard M. D., et al. Methamphetamine use and cardiovascular disease. Arterioscler Thromb Vasc Biol 2019;39(9):1739-46. [PMID: 31433698]
Lerner A., Klein M. Dependence, withdrawal and rebound of CNS drugs: an update and regulatory considerations for new drugs development. Brain Commun 2019;1(1):fcz025. [PMID: 32954266]
Levin F. R., Mariani J. J., Pavlicova M., et al. Extended release mixed amphetamine salts and topiramate for cocaine dependence: a randomized clinical replication trial with frequent users. Drug Alcohol Depend 2020;206:107700. [PMID: 31753736]
Mariani J. J., Pavlicova M., Bisaga A., et al. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol Psychiatry 2012;72(11):950-56. [PMID: 22795453]
Maricich Y. A., Nunes E. V., Campbell A. N., et al. Safety and efficacy of a digital therapeutic for substance use disorder: secondary analysis of data from a NIDA clinical trials network study. Subst Abus 2022;43(1):937-42. [PMID: 35420979]
McKetin R., Leung J., Stockings E., et al. Mental health outcomes associated with of the use of amphetamines: a systematic review and meta-analysis. EClinicalMedicine 2019;16:81-97. [PMID: 31832623]
McKetin R., Lubman D. I., Baker A. L., et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry 2013;70(3):319-24. [PMID: 23303471]
NIDA. Drug overdose death rates. 2023 Jun 30. https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates [accessed 2021 Dec 20]
Nuijten M., Blanken P., van de Wetering B., et al. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. Lancet 2016;387(10034):2226-34. [PMID: 27015909]
NYC Health. Epi data brief: unintentional drug poisoning (overdose) deaths in New York City in 2020. 2021 Nov. https://www.nyc.gov/assets/doh/downloads/pdf/epi/databrief129.pdf [accessed 2022 Dec 19]
NYSDOH. New York State opioid annual data report 2021: Figure 1.9 Overdose deaths involving cocaine with and without synthetic opioids (other than methadone), New York State, 2010-2019. 2023 May 30. https://www.health.ny.gov/statistics/opioid/data/pdf/nys_opioid_annual_report_2021.pdf [accessed 2022 Dec 20]
Pani P. P., Trogu E., Vacca R., et al. Disulfiram for the treatment of cocaine dependence. Cochrane Database Syst Rev 2010;(1):CD007024. [PMID: 20091613]
Paulus M. P., Stewart J. L. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry 2020;77(9):959-66. [PMID: 32267484]
Reddy P. K., Ng T. M., Oh E. E., et al. Clinical characteristics and management of methamphetamine-associated cardiomyopathy: state-of-the-art review. J Am Heart Assoc 2020;9(11):e016704. [PMID: 32468897]
Riley A. L., Nelson K. H., To P., et al. Abuse potential and toxicity of the synthetic cathinones (i.e., "bath salts"). Neurosci Biobehav Rev 2020;110:150-73. [PMID: 31101438]
SAMHSA. Treatment for stimulant use disorders. 2021 Oct 13. https://store.samhsa.gov/sites/default/files/pep21-02-01-004.pdf [accessed 2023 May 1]
SAMHSA. 2021 National Survey on Drug Use and Health (NSDUH) annual national report. 2023 Jan 4. https://www.samhsa.gov/data/report/2021-nsduh-annual-national-report [accessed 2023 May 1]
Schreck B., Victorri-Vigneau C., Guerlais M., et al. Slam practice: a review of the literature. Eur Addict Res 2021;27(3):161-78. [PMID: 33279895]
Semple S. J., Strathdee S. A., Zians J., et al. Factors associated with experiences of stigma in a sample of HIV-positive, methamphetamine-using men who have sex with men. Drug Alcohol Depend 2012;125(1-2):154-59. [PMID: 22572209]
Sessa B., Higbed L., Nutt D. A review of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry 2019;10:138. [PMID: 30949077]
Smid M. C., Metz T. D., Gordon A. J. Stimulant use in pregnancy: an under-recognized epidemic among pregnant women. Clin Obstet Gynecol 2019;62(1):168-84. [PMID: 30601144]
Stone E. M., Kennedy-Hendricks A., Barry C. L., et al. The role of stigma in U.S. primary care physicians' treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Strong C., Huang P., Li C. W., et al. HIV, chemsex, and the need for harm-reduction interventions to support gay, bisexual, and other men who have sex with men. Lancet HIV 2022;9(10):e717-25. [PMID: 35926550]
Tomkins A., George R., Kliner M. Sexualised drug taking among men who have sex with men: a systematic review. Perspect Public Health 2019;139(1):23-33. [PMID: 29846139]
Trivedi M. H., Walker R., Ling W., et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med 2021;384(2):140-53. [PMID: 33497547]
Tsai A. C., Kiang M. V., Barnett M. L., et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
van Boekel L. C., Brouwers E. P., van Weeghel J., et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
Zarse E. M., Neff M. R., Yoder R., et al. The adverse childhood experiences questionnaire: two decades of research on childhood trauma as a primary cause of adult mental illness, addiction, and medical diseases. Cogent Medicine 2019;6(1):1581447. https://doi.org/10.1080/2331205X.2019.1581447
Zweben J. E., Cohen J. B., Christian D., et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004;13(2):181-90. [PMID: 15204668]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | July 03, 2023 |
| Date of current publication | September 26, 2023 |
| Highlights of changes, additions, and updates in the September 26, 2023 edition |
— |
| Intended users | NYS clinicians |
| Lead author(s) |
Judith Griffin, MD; Sara Lorenz Taki, MD; Timothy J. Wiegand, MD |
| Writing group |
Susan D. Whitley, MD; Sharon L. Stancliff, MD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD |
| Author and writing group conflict of interest disclosures | There are no author or writing group conflict of interest disclosures |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines | |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on October 27, 2023

