Purpose of This Guideline
Date of current publication: April 17, 2023
Lead author: David E. Bernstein, MD
Writing group: Joshua S. Aron, MD; Christine A. Kerr, MD; Colleen Flanigan, RN, MS; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Hepatitis C Virus (HCV) Guideline Committee
Date of original publication: July 31, 2017
This guideline for treatment of chronic hepatitis C virus (HCV) infection was developed by the New York State Department of Health AIDS Institute (NYSDOH AI) to guide primary care providers and other practitioners in New York State in treating patients with chronic HCV infection. The guideline aims to achieve the following goals:
- Provide clinicians with current clinical evidence-based recommendations on treating and curing chronic HCV to 1) increase the number of New York State residents treated for and cured of chronic HCV and 2) reduce the growing burden of morbidity and mortality associated with chronic HCV infection.
- Educate clinicians on safely and correctly prescribing anti-HCV medications.
- Educate clinicians on the effects of HCV infection during pregnancy and the risk of vertical HCV transmission during the perinatal period.
- Advise clinicians on the risks associated with HCV treatment in pregnant individuals.
- Provide evidence-based clinical recommendations to support the goals of the New York State Hepatitis C Elimination Plan (NY Cures HepC).
Treatment and cure of chronic HCV: The availability of safe and effective regimens of oral direct-acting antivirals (DAAs) revolutionized HCV care, and DAA regimens are the standard of care for treating and curing chronic HCV. DAAs are molecules that work at different stages of the HCV lifecycle, targeting and inhibiting specific nonstructural proteins of HCV to disrupt viral replication and infection UpToDate 2021. The classes of DAAs are defined by their mechanism of action and therapeutic target.
Current DAAs for treatment of chronic HCV:
- Protease inhibitors (-previrs): glecaprevir, voxilaprevir, grazoprevir
- NS5A inhibitors (-asvirs): ledipasvir, velpatasvir, pibrentasvir, elbasvir
- NS5B nucleoside polymerase inhibitor (-buvir): sofosbuvir
The goal of HCV therapy is a sustained virologic response (SVR), which is defined as the absence of detectable HCV RNA at least 12 weeks after treatment completion. An SVR is the equivalent of a cure. DAA regimens have been associated with an SVR rate of ≥90% and have excellent tolerability in both treatment-naive and treatment-experienced patients with and without cirrhosis Falade-Nwulia, et al. 2017.
See the tables in the guideline section Recommended DAA Treatment Regimens for options for initial treatment of chronic HCV or retreatment of chronic HCV after treatment failure.
HCV Treatment Goals and Considerations
| RECOMMENDATIONS |
|
Considerations in HCV Treatment
Contraindications
|
Abbreviations: CrCl, creatinine clearance; DAA, direct-acting antiviral; HBV, hepatitis B virus; HCV, hepatitis C virus; RBV, ribavirin. |
Goals
The goal of treatment in patients with chronic HCV infection is to attain a virologic cure, as evidenced by a sustained viral response, in order to reduce all-cause mortality and liver-related complications, including end-stage liver disease, hepatocellular carcinoma, and the morbidity and mortality associated with the extrahepatic manifestation of chronic HCV infection. With the significant advances in treatment, all patients with chronic HCV infection, regardless of fibrosis stage, are considered candidates for antiviral therapy Simmons, et al. 2015; Smith-Palmer, et al. 2015; van der Meer, et al. 2012.
| KEY POINTS |
|
Considerations
This guideline includes recommendations for treating patients with chronic HCV infection, with consideration of individual characteristics such as viral genotype, presence of cirrhosis, and previous treatment history. Concurrent medical conditions, potential drug-drug interactions, and cost/coverage influence are factors in selecting HCV treatment regimens; sex, age, viral load levels, substance use disorders, mental health disorders, and HIV coinfection are not factors in choosing regimens.
The tables in the guideline section Recommended DAA Treatment Regimens present several options for treatment in each category. No regimen is prioritized or recommended over another; regimens are listed alphabetically.
| KEY POINT |
|
Renal impairment: For patients with a CrCl <50 mL/min, RBV should be used with caution; if used, a reduced dose of 200 mg per day is recommended FDA 2011. No dose adjustment of ledipasvir/sofosbuvir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, or glecaprevir/pibrentasvir is required in patients with mild, moderate, or severe renal impairment FDA(a) 2019; FDA(b) 2019; FDA 2017; FDA(a) 2016.
Resistance testing: At present, testing for resistance-associated substitutions (RASs) is not universally recommended. RASs are also referred to as resistance analysis populations and resistance-associated variants. RAS testing is performed when retreatment is considered for patients for whom treatment with a DAA regimen containing an NS5A or NS5B inhibitor has failed (see guideline section Recommended DAA Regimens After PEG-IFN Treatment Failure).
NS5A testing is recommended in patients with HCV genotype 3 who are being considered for 12 weeks of sofosbuvir/velpatasvir and are treatment-naive and have cirrhosis or are treatment-experienced Hezode, et al. 2018; Foster, et al. 2015. If the Y93H RAS is present, weight-based RBV should be added to the regimen or another regimen should be selected.
For more information on HCV resistance, see the Infectious Diseases Society of America/American Association for the Study of Liver Disease HCV Resistance Primer.
HCV Testing and Management in Pregnant Adults
| RECOMMENDATIONS |
HCV Testing and Management in Pregnant Adults
Contraceptive Use With HCV Treatment Containing RBV
|
Abbreviations: AASLD, American Association for the Study of Liver Diseases; CDC, Centers for Disease Control and Prevention; DAA, direct-acting antiviral; HCV, hepatitis C virus; IDSA, Infectious Diseases Society of America; RBV, ribavirin. |
HCV screening during pregnancy: In New York State, excluding New York City, 2,416 cases of HCV were reported in 2020 among individuals of childbearing age, 15 to 44 years old NYSDOH 2022. In New York City, in 2020, 447 cases of HCV were reported among individuals of childbearing age NYCDOHMH 2021.
These data raise concerns about reaching and treating this population and the potential for perinatal HCV transmission. Data indicate that in areas of high HCV prevalence, 10% to 28% of pregnant individuals with HCV infection are not identified through risk-based screening Andes, et al. 2021; Koniares, et al. 2020; Fernandez, et al. 2016; Waruingi, et al. 2015; Thomas 2013. Thus, in alignment with Centers for Disease Control and Prevention Schillie, et al. 2020 and American College of Obstetricians and Gynecologists (ACOG) ACOG 2022 recommendations, the NYSDOH and this committee recommend universal testing for HCV infection in individuals who are pregnant or planning to become pregnant and that screening be repeated with each pregnancy. Identifying HCV presents an opportunity to ensure linkage to care and guide obstetric clinicians on the maternal and fetal risks in pregnant patients with HCV. In addition, universal HCV testing during pregnancy appears to be cost-effective Chaillon, et al. 2021; Tasillo, et al. 2019.
| KEY POINTS |
|
HCV infection during pregnancy: There are no published large-scale studies on DAA treatment for HCV during pregnancy, and treatment of pregnant individuals is not currently recommended. Clinical trials are underway to evaluate the use of DAAs for the treatment of HCV during pregnancy Chappell, et al. 2020; Yattoo 2018, and the clinician could discuss the possibility of clinical trial participation and refer the patient as appropriate (see Clinical Trials.gov).
If an individual becomes pregnant during DAA treatment, the clinician and patient should discuss the risks (e.g., no information on the effects of the medication on the fetus) and benefits (e.g., probable HCV cure) of continuing treatment and refer the patient to a specialist experienced in managing HCV in pregnancy, such as a hepatologist, gastroenterologist, infectious disease specialist, or high-risk obstetrician. Clinicians with patients who have been exposed to DAA treatment during pregnancy can contact the Treatment in Pregnancy for Hepatitis C Registry.
HCV infection, compared with no HCV infection, is associated with a higher incidence of intrahepatic cholestasis in pregnancy. Intrahepatic cholestasis in pregnancy has significant maternal and fetal morbidity Wijarnpreecha, et al. 2017, and patients with HCV and this condition should be followed by a liver specialist or an obstetrician experienced in managing high-risk pregnancies Wijarnpreecha, et al. 2017. HCV infection during pregnancy has been associated with other adverse maternal and fetal outcomes, including gestational diabetes, low birth weight, small for gestational age, impaired intrauterine fetal growth, preterm delivery, miscarriage, and congenital anomalies Connell et al. 2011. Researchers note that the specific role of HCV in determining these outcomes is unclear because the data may be confounded by comorbid polysubstance use Connell, et al. 2011. Patients with HCV and recent or active substance use during pregnancy should be referred to care providers experienced in managing substance use during pregnancy for evaluation, treatment, and harm reduction services.
Perinatal transmission: Approximately 1.0% to 3.6% of pregnant individuals in the United States have HCV infection Edlin, et al. 2015; Floreani 2013, and the risk of perinatal transmission is estimated at 6% for patients with HCV monoinfection and >10% for patients with HIV/HCV coinfection Pawlowska 2015; Arshad, et al. 2011. Currently, no antiviral treatment is available to reduce HCV transmission during pregnancy.
Intrauterine, intrapartum, and postpartum HCV transmission to the fetus have been reported, but postpartum transmission is believed to be rare Gibb, et al. 2000. In utero transmission may occur during all 3 trimesters, and the risk of transmission may be associated with high maternal HCV RNA levels Elrazek, et al. 2017. When an individual’s immune response is altered during pregnancy, HCV RNA levels usually increase during the second and third trimesters, and there is a synchronous decrease in maternal alanine transaminase levels Gervais, et al. 2000. HCV RNA levels decline after delivery; spontaneous postpartum clearance of the HCV infection has been reported and should be considered when evaluating postpartum patients for treatment Hashem, et al. 2017; Prasad and Honegger 2013.
Data are limited on intrauterine HCV transmission during invasive procedures, such as fetal scalp monitoring, intrauterine pressures, chorionic villi sampling, and amniocentesis. Conditions such as premature rupture of membranes during pregnancy have been associated with increased risk of HCV transmission Mast, et al. 2005. However, observational studies have demonstrated that mode of delivery (Cesarean section [C-section] or vaginal) is not associated with the rate of perinatal HCV transmission Ghamar Chehreh, et al. 2011; Mast, et al. 2005; European Paediatric Hepatitis C Virus Network 2001. The Society for Maternal-Fetal Medicine/ACOG guidelines recommend against performing a C-section simply to reduce the risk of HCV transmission Hughes, et al. 2017; Cottrell, et al. 2013.
Breastfeeding: For postpartum individuals with HCV, breastfeeding is an option and is not associated with an increased risk of HCV transmission to the infant Cottrell et al. 2013. However, it should be noted that if the postpartum individual has cracked or bleeding nipples, HCV transmission may occur during breastfeeding through blood or nonintact skin exposure CDC 2021. Early discussion with lactation consultants during or after pregnancy may be helpful to minimize difficulties with breastfeeding. For pregnant patients with HIV/HCV coinfection, clinicians should consult ACOG: Labor and Delivery Management of Women With Human Immunodeficiency Virus Infection.
Contraceptive use with HCV treatment containing RBV: For all female and male patients planning conception within 6 months of treatment, use of RBV is contraindicated due to the teratogenic effects of the drug Sinclair, et al. 2017; FDA 2011. Before prescribing an RBV-containing regimen for a patient of childbearing potential, a negative pregnancy test is required immediately before initiation of therapy, and using 2 forms of contraception or abstinence is advised during therapy and for 6 months after. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of therapy in female patients and female partners of male patients taking RBV.
If an individual with HCV becomes pregnant while taking an HCV treatment regimen containing RBV, RBV should be discontinued.
Recommended DAA Treatment Regimens
| RECOMMENDATIONS |
Recommended DAA Treatment Regimens
|
Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; PEG-IFN, pegylated interferon; RAS, resistance-associated substitution; RBV, ribavirin. |
All regimens listed in this guideline were available as of October 2022.
These recommendations for treatment of chronic HCV in adults ≥18 years old were developed by the NYSDOH AI HCV Guideline Committee to guide primary care providers and other clinicians in New York State in treating patients with chronic HCV infection. Treatment guidelines for patients ≤17 years old are available at the American Association of the Study of Liver Diseases/Infectious Disease Society of America HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. All available DAA regimens are pangenotypic. As such, these recommendations are based on a patient’s treatment experience instead of genotype.
HIV/HCV coinfection: Recommendations for treatment of chronic HCV infection in patients with HIV are the same as those for patients who do not have HIV, but attention to potential drug-drug interactions between DAAs and antiretrovirals is needed (see Box 1, below). Clinicians are encouraged to consult a specialist in the treatment of liver disease or viral hepatitis and an experienced HIV care provider as needed.
| KEY POINTS |
|
Undetectable or indeterminate genotype: Rarely, laboratories report the results of an HCV genotype test as “undetectable” or “indeterminate” for a patient with detectable HCV viral load Germer, et al. 2011. These HCV genotype reports are consistent with active HCV infection. The laboratory may be able to clarify the specific reason for the result. For example, an “undetectable” result may be due to the lower sensitivity of the genotype test compared with the HCV RNA test or a level of HCV RNA that is too low to perform the assay for genotype.
Data on treating patients with HCV who have an undetectable or indeterminate genotype are limited. Patients who have an undetectable or indeterminate HCV genotype can be treated with a pangenotypic regimen such as glecaprevir/pibrentasvir or sofosbuvir/velpatasvir.
Table 1, below, lists recommended oral DAAs. All regimens listed in drug regimen tables for all HCV genotypes refer to oral medications.
Notes:
|
|
| Table 1: Recommended Oral Direct-Acting Antiviral Drug Regimens for Adults [a] With Chronic HCV (October 2022) | |
| Drug/Combination | Trade Name |
| Glecaprevir/pibrentasvir | Mavyret [b] |
| Ledipasvir/sofosbuvir | Multiple brands [c] |
| Sofosbuvir/velpatasvir | Multiple brands |
| Sofosbuvir/velpatasvir/voxilaprevir | Vosevi |
Drug-drug interactions: It is essential to check current resources for potential drug-drug interactions before prescribing direct-acting antiviral (DAA) therapy for hepatitis C virus (HCV) treatment.
| Box 1: Online Resources for Identifying Drug-Drug Interactions Associated With DAAs |
|
Recommended Treatment Regimens for Treatment-Naive Patients
Recommended regimens: The recommendations are organized by whether or not the patient has compensated cirrhosis. All drugs in the recommended regimens listed below are oral medications.
Treatment interruption and adherence: To achieve HCV cure, strict adherence to the medications as prescribed is essential. Before initiating treatment with a DAA regimen, develop an adherence plan with the patient, address potential barriers, and make support available if it is needed. Clinicians are advised to consult an HCV treatment specialist if a patient’s DAA treatment is interrupted.
Drug names: A “/” between 2 drug names indicates a co-formulated tablet.
Rating of regimens: All regimen choices listed below are rated A1 (strong recommendation, with high-quality evidence from at least 1 randomized trial with clinical outcomes or validated laboratory endpoints) except where indicated.
| Abbreviations: HCV, hepatitis C virus; RAS, resistance-associated substitution; RBV, ribavirin.
Notes:
|
|||
| Table 2: Preferred Regimens for HCV Treatment-Naive Patients |
|||
| Genotype | Regimen | Treatment Duration | |
| No Cirrhosis |
Compensated Cirrhosis |
||
| 1a, 1b, 2, 4, 5, 6 | Glecaprevir 300 mg/pibrentasvir 120 mg once daily | 8 weeks [a] | 8 weeks [b] |
| Sofosbuvir 400 mg/velpatasvir 100 mg once daily | 12 weeks [c] | 12 weeks [c] | |
| 3 | Glecaprevir 300 mg/pibrentasvir 120 mg once daily | 12 weeks [d] | 12 weeks [e] |
| Sofosbuvir 400 mg/velpatasvir 100 mg once daily | 12 weeks [f] | 12 weeks [g,h] | |
Download Table 2: Preferred Regimens for HCV Treatment-Naive Patients Printable PDF
| Abbreviation: HCV, hepatitis C virus.
Notes: |
|||
| Table 3: Alternative Regimens for HCV Treatment-Naive Patients |
|||
| Genotype/Patient Characteristics | Regimen | Treatment Duration | |
| No Cirrhosis |
Compensated Cirrhosis |
||
| 1a or 1b, non-Black, HIV-negative, HCV RNA <6 mil copies/mL (A2) | Ledipasvir 90 mg/sofosbuvir 400 mg once daily | 8 weeks [a] | 12 weeks [b] |
| 1a or 1b, Black, HIV-positive or HCV RNA >6 mil copies/mL (A2) | Ledipasvir 90 mg/sofosbuvir 400 mg once daily | 12 weeks [a] | 12 weeks [c] |
| 4, 5, 6 | Ledipasvir 90 mg/sofosbuvir 400 mg once daily | 12 weeks [d] | 12 weeks [d] |
Download Table 3: Alternative Regimens for HCV Treatment-Naive Patients Printable PDF
Recommended DAA Regimens After PEG-IFN Treatment Failure
Recommended regimens: The recommendations are organized by whether or not the patient has compensated cirrhosis. All drugs in the recommended regimens listed below are oral medications.
Treatment interruption and adherence: To achieve HCV cure, strict adherence to the medications as prescribed is essential. Before initiating treatment with a DAA regimen, develop an adherence plan with the patient, address potential barriers, and make support available if it is needed. Clinicians are advised to consult an HCV treatment specialist if a patient’s DAA treatment is interrupted.
Drug names: A “/” between 2 drug names indicates a co-formulated tablet.
Rating of regimens: All regimen choices listed below are rated A1 (strong recommendation, with high-quality evidence from at least 1 randomized trial with clinical outcomes or validated laboratory endpoints) except where indicated.
| Abbreviations: PEG-IFN, pegylated interferon; RAS, resistance-associated substitution; RBV, ribavirin.
Notes:
|
|||
| Table 4: Preferred Regimens After PEG-IFN Plus RBV Treatment Failure | |||
| Genotype | Regimen | Treatment Duration | |
| No Cirrhosis |
Compensated Cirrhosis |
||
| 1a, 1b, 2, 4, 5, 6 | Glecaprevir 300 mg/pibrentasvir 120 mg once daily | 8 weeks [a] | 12 weeks [b] |
| Sofosbuvir 400 mg/velpatasvir 100 mg once daily | 12 weeks [c] | 12 weeks [c] | |
| 3 | Glecaprevir 300 mg/pibrentasvir 120 mg once daily | 16 weeks [a] | 16 weeks [b] |
| Sofosbuvir 400 mg/velpatasvir 100 mg once daily | 12 weeks [d] | 12 weeks [d,e] | |
Download Table 4: Preferred Regimens After PEG-IFN Plus RBV Treatment Failure Printable PDF
| Abbreviations: PEG-IFN, pegylated interferon; RBV, ribavirin.
Notes: |
|||
| Table 5: Alternative Regimens After PEG-IFN Plus RBV Treatment Failure | |||
| Genotype | Regimen | Treatment Duration | |
| No Cirrhosis |
Compensated Cirrhosis |
||
| 1a, 1b | Ledipasvir 90 mg/sofosbuvir 400 mg once daily | 12 weeks [a] | 12 weeks [a] |
| Ledipasvir 90 mg/sofosbuvir 400 mg once daily plus weight-based RBV twice daily | Not indicated | 12 weeks [a] | |
| 4, 5, 6 | Ledipasvir 90 mg/sofosbuvir 400 mg once daily | 12 weeks [b] | 12 weeks [b] |
Download Table 5: Alternative Regimens After PEG-IFN Plus RBV Treatment Failure Printable PDF
Recommended DAA Retreatment Regimens
Recommended regimens: The recommendations are organized by whether or not the patient has compensated cirrhosis. All drugs in the recommended regimens listed below are oral medications.
Treatment interruption and adherence: To achieve HCV cure, strict adherence to the medications as prescribed is essential. Before initiating treatment with a DAA regimen, develop an adherence plan with the patient, address potential barriers, and make support available if it is needed. Clinicians are advised to consult an HCV treatment specialist if a patient’s DAA treatment is interrupted.
Drug names: A “/” between 2 drug names indicates a co-formulated tablet.
Rating of regimens: All regimen choices listed below are rated A1 (strong recommendation, with high-quality evidence from at least 1 randomized trial with clinical outcomes or validated laboratory endpoints) except where indicated.
| Abbreviations: DAA, direct-acting antiviral; RBV, ribavirin.
Notes:
|
|||
| Table 6: Recommended Regimens After Sofosbuvir or Elbasvir/Grazoprevir Treatment Failure | |||
| Genotype | Regimen | Treatment Duration | |
| No Cirrhosis |
Compensated Cirrhosis |
||
| 1a, 1b, 2, 4, 5, 6 | Glecaprevir 300 mg/pibrentasvir 120 mg once daily | 16 weeks [a,b] | 16 weeks [b] |
| Sofosbuvir 400 mg/velpatasvir 100 mg/voxilaprevir 100 mg once daily | 12 weeks [c] | 12 weeks [c] | |
| 3 | Sofosbuvir 400 mg/velpatasvir 100 mg/voxilaprevir 100 mg once daily plus weight-based RBV twice daily | 12 weeks [d] | 12 weeks [d] |
| Abbreviation: RBV, ribavirin.
Notes:
|
|||
| Table 7: Recommended Regimens After Glecaprevir/Pibrentasvir Treatment Failure | |||
| Genotype | Regimen | Treatment Duration | |
| No Cirrhosis |
Compensated Cirrhosis |
||
| 1a, 1b, 2, 3, 4, 5, 6 | Glecaprevir 300 mg/pibrentasvir 120 mg plus sofosbuvir 400 mg once daily plus weight-based RBV twice daily | 16 weeks [a] | 16 weeks [b] |
| Sofosbuvir 400 mg/velpatasvir 100 mg/voxilaprevir 100 mg once daily | 12 weeks [c] | 12 weeks [d,e] | |
Monitoring During DAA Treatment
| RECOMMENDATIONS |
Monitoring of Patients Taking RBV
Monitoring for HBV Reactivation
|
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; HBsAg, HBV surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; RBV, ribavirin. |
The adverse events associated with direct-acting antiviral (DAA) treatment are listed in Table 8, below, and most are manageable. Patients who are taking RBV and experience insomnia may need to adjust the timing of the dose to earlier in the afternoon to avoid any sleep disruption.
| KEY POINT |
|
Transient transaminase and bilirubin elevations may occur during the normal course of DAA therapy. However, severe laboratory value elevations and rare hepatic decompensation have been reported with protease inhibitors during the treatment of patients with cirrhosis FDA(b) 2019; FDA 2017; FDA(b) 2016; Hayashi, et al. 2016. Therefore, if the ALT level is elevated above baseline 4 weeks after treatment is initiated, testing should be repeated and levels monitored according to the drug’s prescribing information FDA(b) 2019; FDA 2017; FDA(b) 2016; Hayashi, et al. 2016.
HBV reactivation and HBV-related hepatic flares have occurred both during and after DAA therapy in patients not receiving HBV treatment Wang, et al. 2017; Sulkowski, et al. 2016; Collins, et al. 2015; Ende, et al. 2015. The U.S. Food and Drug Administration has issued a drug safety warning regarding these risks.
| Sources: FDA(a) 2019; FDA(b) 2019; FDA 2017; FDA(a) 2016; FDA(b) 2016; Hayashi, et al. 2016; FDA 2011 | |
| Table 8: Adverse Events Associated with Direct-Acting Antivirals | |
| Drug or Combination (brand name) |
Most Common Adverse Reactions (proportion observed) |
| Glecaprevir/pibrentasvir (GLE/PIB; Mavyret) |
Headache and fatigue (>10%) |
| Ledipasvir/sofosbuvir (LED/SOF; Harvoni; multiple brands) |
Asthenia, headache, and fatigue (≥10%) |
| Ribavirin (Copegus) |
Fatigue/asthenia, pyrexia, myalgia, and headache in adults receiving combination therapy (>40%) |
| Sofosbuvir/velpatasvir (SOF/VEL; Epclusa; multiple brands) |
|
| Sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX; Vosevi) |
Headache, fatigue, diarrhea, and nausea (≥10%) |
Post-Treatment Care
| RECOMMENDATIONS |
Evaluating the Response to HCV Treatment
Post-Treatment Monitoring
Patients With Persistent Liver Disease
|
Abbreviations: DAA, direct-acting antiviral; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; RBV, ribavirin; SVR, sustained viral response. |
After treatment for chronic HCV infection, follow-up care is based on individual patient factors, including response to recent treatment, previous treatment history, degree of hepatic fibrosis, comorbidities, and cofactors for other sources of liver injury, such as alcohol use or fatty liver disease.
Evaluating the Response to HCV Treatment
All treated individuals should have HCV RNA testing performed 12 weeks after treatment. If there is no detectable HCV RNA at 12 weeks, HCV infection has been cured. In the absence of recurrent risk factors, subsequent HCV testing is not required. However, with late relapse reported in rare (<0.5%) cases, some clinicians may choose to retest at 24 and/or 48 weeks after the end of treatment Jacobson, et al. 2017.
Successful treatment of chronic HCV infection results in no detectable HCV RNA, but antibodies to HCV are typically retained for life. It is important for treated individuals to understand that they will continue to have antibodies but not active HCV infection. It is also important for patients to understand that, although antibodies to HCV will continue to be present after treatment, HCV antibodies do not offer protection from HCV reinfection. All individuals with no detectable HCV RNA are susceptible to reinfection if re-exposed to HCV. Although the overall rate of reinfection is low, it is elevated among populations at higher risk Martinello, et al. 2017. A meta-analysis of 59 studies reporting recurrence after an SVR in 9,049 patients found that the summary 5-year risk of HCV reinfection among high-risk populations was 10.67% Simmons, et al. 2016. High risk was defined as having 1 or more risk factors, currently or formerly, for reinfection (injection drug use, imprisonment, and being a man who has sex with other men). Among low-risk populations, defined as those with no known risk factors, the summary 5-year recurrence risk was 0.95% Simmons, et al. 2016. For discussion of risk factors, see the NYSDOH AI guideline Hepatitis C Virus Screening, Testing, and Diagnosis in Adults.
Post-Treatment Monitoring
It is important to monitor the resolution of patients’ HCV treatment-related adverse events. RBV-containing regimens are teratogenic; patients receiving RBV-containing regimens and their partners should be counseled to avoid pregnancy during treatment and up to 6 months post-treatment. Two forms of effective birth control should be used FDA 2011.
Table 8: Adverse Events Associated with Direct-Acting Antivirals provides a list of adverse events associated with DAA regimens. During treatment with RBV, patients may experience hemolytic anemia, nausea, cough, shortness of breath, rash, dry skin, pruritus, lactic acidosis, or pancreatitis FDA 2011. Patients should be monitored through the follow-up period for resolution of any symptoms.
Hepatitis B virus (HBV) reactivation: HBV-related hepatic flares have been reported during and after DAA therapy in patients who were not receiving concurrent HBV treatment Wang, et al. 2017; De Monte, et al. 2016; Hayashi, et al. 2016; Sulkowski, et al. 2016; Takayama, et al. 2016; Collins, et al. 2015; Ende, et al. 2015. The U.S. Food and Drug Administration has issued a drug safety warning regarding these risks. Although data are insufficient to make a definitive recommendation regarding monitoring in patients with isolated hepatitis B core antibody AASLD/IDSA 2021, it is important to consider HBV reactivation as part of the differential diagnosis for patients with HBV infection who experience unexplained increases in liver enzymes during or after completion of DAA treatment.
Patients With Persistent Liver Disease
Cessation of fibrosis progression and histological improvement are among the benefits of treating chronic HCV infection. However, patients should still be monitored for potential post-treatment decompensation Jacobson, et al. 2017. Individuals cured of HCV infection remain at risk of liver disease progression if they have advanced baseline fibrosis, other chronic liver conditions (e.g., chronic HBV, non-alcoholic fatty liver disease), comorbidities (e.g., metabolic syndrome, alcohol use, uncontrolled coinfection with HIV), or at risk of liver injury from drugs or dietary supplements Vandenbulcke, et al. 2016.
There is wide individual variation in the time needed for fibrosis progression in patients with chronic liver disease. It is important to maintain an elevated suspicion for progression and the complications associated with hepatic decompensation, particularly in individuals with bridging fibrosis or cirrhosis before the initiation of DAA therapy and HCV cure.
In patients with bridging fibrosis or cirrhosis, an ultrasound and alpha-fetoprotein testing should be performed every 6 months, regardless of SVR, to screen for HCC Jacobson, et al. 2017. The risk of HCC for patients with stage 3 or higher fibrosis is 1.5% to 5% per year, but it is not known whether the histologic improvement after successful treatment mitigates this risk Bruix and Sherman 2011.
All Recommendations
| ALL RECOMMENDATIONS: TREATMENT OF CHRONIC HEPATITIS C VIRUS INFECTION IN ADULTS |
|
Considerations in HCV Treatment
Contraindications
HCV Testing and Management in Pregnant Adults
Contraceptive Use With HCV Treatment Containing RBV
Recommended DAA Treatment Regimens
Monitoring of Patients Taking RBV
Monitoring for HBV Reactivation
Evaluating the Response to HCV Treatment
Post-Treatment Monitoring
Patients With Persistent Liver Disease
|
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, alanine transaminase; AST, aspartate aminotransferase; CDC, Centers for Disease Control and Prevention; CrCl, creatinine clearance; DAA, direct-acting antiviral; HBsAg, HBV surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IDSA, Infectious Diseases Society of America; PEG-IFN, pegylated interferon; RAS, resistance-associated substitution; RBV, ribavirin; SVR, sustained viral response. |
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
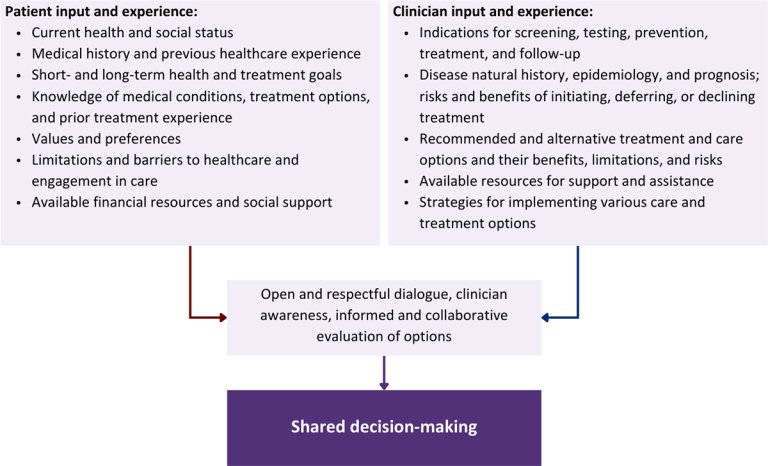
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
AASLD/IDSA. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. 2021 Oct. https://www.hcvguidelines.org/ [accessed 2022 Aug 29]
Abergel A., Asselah T., Metivier S., et al. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis 2016;16(4):459-64. [PMID: 26803446]
ACOG. Routine hepatitis C virus screening in pregnant individuals. 2022 Jan. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/05/routine-hepatitis-c-virus-screening-in-pregnant-individuals [accessed 2022 Aug 29]
Afdhal N., Reddy K. R., Nelson D. R., et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370(16):1483-93. [PMID: 24725238]
Andes A., Ellenberg K., Vakos A., et al. Hepatitis C virus in pregnancy: a systematic review of the literature. Am J Perinatol 2021;38(S 01):e1-13. [PMID: 32323289]
Arshad M., El-Kamary S. S., Jhaveri R. Hepatitis C virus infection during pregnancy and the newborn period--are they opportunities for treatment?. J Viral Hepat 2011;18(4):229-36. [PMID: 21392169]
Asselah T., Kowdley K. V., Zadeikis N., et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol 2018;16(3):417-26. [PMID: 28951228]
Bourliere M., Gordon S. C., Flamm S. L., et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med 2017;376(22):2134-46. [PMID: 28564569]
Bourliere M., Gordon S. C., Schiff E. R., et al. Deferred treatment with sofosbuvir-velpatasvir-voxilaprevir for patients with chronic hepatitis C virus who were previously treated with an NS5A inhibitor: an open-label substudy of POLARIS-1. Lancet Gastroenterol Hepatol 2018;3(8):559-65. [PMID: 29859740]
Brown R. S., Buti M., Rodrigues L., et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1-6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol 2019;72(3):441-49. [PMID: 31682879]
Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53(3):1020-22. [PMID: 21374666]
CDC. Breastfeeding: hepatitis B or C infections. 2021 Aug 10. https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/maternal-or-infant-illnesses/hepatitis.html [accessed 2022 Aug 29]
Chaillon A., Wynn A., Kushner T., et al. Cost-effectiveness of antenatal rescreening among pregnant women for hepatitis C in the United States. Clin Infect Dis 2021;73(9):e3355-57. [PMID: 32282879]
Chappell C. A., Scarsi K. K., Kirby B. J., et al. Ledipasvir plus sofosbuvir in pregnant women with hepatitis C virus infection: a phase 1 pharmacokinetic study. Lancet Microbe 2020;1(5):e200-208. [PMID: 32939459]
Collins J. M., Raphael K. L., Terry C., et al. Hepatitis B virus reactivation during successful treatment of hepatitis C virus with sofosbuvir and simeprevir. Clin Infect Dis 2015;61(8):1304-6. [PMID: 26082511]
Connell L. E., Salihu H. M., Salemi J. L., et al. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int 2011;31(8):1163-70. [PMID: 21745298]
Cottrell E. B., Chou R., Wasson N., et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158(2):109-13. [PMID: 23437438]
De Monte A., Courjon J., Anty R., et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol 2016;78:27-30. [PMID: 26967675]
Edlin B. R., Eckhardt B. J., Shu M. A., et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015;62(5):1353-63. [PMID: 26171595]
Elrazek A., Amer M., El-Hawary B., et al. Prediction of HCV vertical transmission: what factors should be optimized using data mining computational analysis. Liver Int 2017;37(4):529-33. [PMID: 27125252]
Ende A. R., Kim N. H., Yeh M. M., et al. Fulminant hepatitis B reactivation leading to liver transplantation in a patient with chronic hepatitis C treated with simeprevir and sofosbuvir: a case report. J Med Case Rep 2015;9:164. [PMID: 26215390]
Esteban R., Pineda J. A., Calleja J. L., et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in patients with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology 2018;155(4):1120-27.e4. [PMID: 29958855]
European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. European Paediatric Hepatitis C Virus Network. BJOG 2001;108(4):371-77. [PMID: 11305543]
Falade-Nwulia O., Suarez-Cuervo C., Nelson D. R., et al. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017;166(9):637-48. [PMID: 28319996]
FDA. Copegus (ribavirin) tablets. 2011 Aug. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021511s023lbl.pdf [accessed 2022 Feb 1]
FDA. Vosevi (sofosbuvir, velpatasvir, and voxilaprevir) tablets, for oral use. 2017 Jul. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209195s000lbl.pdf [accessed 2022 Feb 1]
FDA(a). Epclusa (sofosbuvir and velpatasvir) tablets, for oral use. 2016 Jun. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208341s000lbl.pdf [accessed 2022 Feb 1]
FDA(a). Harvoni (ledipasvir and sofosbuvir) tablets, for oral use. 2019 Aug. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212477s000lbl.pdf [accessed 2022 Feb 1]
FDA(b). Zepatier (elbasvir and grazoprevir) tablets, for oral use. 2016 Jan. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208261Orig1s000lbl.pdf [accessed 2022 Feb 1]
FDA(b). Mavyret (glecaprevir and pibrentasvir) tablets, for oral use. 2019 Apr. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209394s006lbl.pdf [accessed 2022 Feb 1]
Feld J. J., Jacobson I. M., Hezode C., et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373(27):2599-2607. [PMID: 26571066]
Fernandez N., Towers C. V., Wolfe L., et al. Sharing of snorting straws and hepatitis C virus infection in pregnant women. Obstet Gynecol 2016;128(2):234-37. [PMID: 27400008]
Floreani A. Hepatitis C and pregnancy. World J Gastroenterol 2013;19(40):6714-20. [PMID: 24187446]
Forns X., Lee S. S., Valdes J., et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 2017;17(10):1062-68. [PMID: 28818546]
Foster G. R., Afdhal N., Roberts S. K., et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015;373(27):2608-17. [PMID: 26575258]
Gane(a) E., Kowdley K. V., Pound D., et al. Efficacy of sofosbuvir, velpatasvir, and GS-9857 in patients with hepatitis C virus genotype 2, 3, 4, or 6 infections in an open-label, phase 2 trial. Gastroenterology 2016;151(5):902-9. [PMID: 27486033]
Gane(b) E., Poordad F., Wang S., et al. High efficacy of ABT-493 and ABT-530 treatment in patients with HCV genotype 1 or 3 infection and compensated cirrhosis. Gastroenterology 2016;151(4):651-59.e1. [PMID: 27456384]
Germer J. J., Mandrekar J. N., Bendel J. L., et al. Hepatitis C virus genotypes in clinical specimens tested at a national reference testing laboratory in the United States. J Clin Microbiol 2011;49(8):3040-43. [PMID: 21613437]
Gervais A., Bacq Y., Bernuau J., et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol 2000;32(2):293-99. [PMID: 10707870]
Ghamar Chehreh M. E., Tabatabaei S. V., Khazanehdari S., et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet 2011;283(2):255-60. [PMID: 20652289]
Gibb D. M., Goodall R. L., Dunn D. T., et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet 2000;356(9233):904-7. [PMID: 11036896]
Hashem M., Jhaveri R., Saleh D. A., et al. Spontaneous viral load decline and subsequent clearance of chronic hepatitis C virus in postpartum women correlates with favorable interleukin-28B gene allele. Clin Infect Dis 2017;65(6):999-1005. [PMID: 28903504]
Hayashi K., Ishigami M., Ishizu Y., et al. A case of acute hepatitis B in a chronic hepatitis C patient after daclatasvir and asunaprevir combination therapy: hepatitis B virus reactivation or acute self-limited hepatitis?. Clin J Gastroenterol 2016;9(4):252-56. [PMID: 27329484]
Hezode C., Reau N., Svarovskaia E. S., et al. Resistance analysis in patients with genotype 1-6 HCV infection treated with sofosbuvir/velpatasvir in the phase III studies. J Hepatol 2018;68(5):895-903. [PMID: 29221887]
Hughes B. L., Page C. M., Kuller J. A. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol 2017;217(5):B2-12. [PMID: 28782502]
Jacobson I. M., Lim J. K., Fried M. W. American Gastroenterological Association Institute clinical practice update-expert review: care of patients who have achieved a sustained virologic response after antiviral therapy for chronic hepatitis C infection. Gastroenterology 2017;152(6):1578-87. [PMID: 28344022]
Kohli A., Kapoor R., Sims Z., et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis 2015;15(9):1049-54. [PMID: 26187031]
Koniares K. G., Fadlallah H., Kolettis D. S., et al. Hepatitis C virus screening in pregnancy. Am J Obstet Gynecol MFM 2020;2(3):100123. [PMID: 33345869]
Kowdley K. V., Gordon S. C., Reddy K. R., et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370(20):1879-88. [PMID: 24720702]
Kowdley K. V., Sundaram V., Jeon C. Y., et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology 2017;65(4):1094-1103. [PMID: 28027579]
Kwo P. Y., Poordad F., Asatryan A., et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol 2017;67(2):263-71. [PMID: 28412293]
Lawitz E., Poordad F. F., Pang P. S., et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet 2014;383(9916):515-23. [PMID: 24209977]
Lok A. S., Sulkowski M. S., Kort J. J., et al. Efficacy of glecaprevir and pibrentasvir in patients with genotype 1 hepatitis C virus infection with treatment failure after NS5A inhibitor plus sofosbuvir therapy. Gastroenterology 2019;157(6):1506-17.e1. [PMID: 31401140]
Martinello M., Grebely J., Petoumenos K., et al. HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat 2017;24(5):359-70. [PMID: 28027424]
Mast E. E., Hwang L. Y., Seto D. S., et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005;192(11):1880-89. [PMID: 16267758]
NYCDOHMH. Hepatitis A, B and C in New York City: 2020 annual report. 2021 Nov 15. https://www1.nyc.gov/assets/doh/downloads/pdf/cd/hepatitis-abc-annual-report-2020.pdf [accessed 2022 Aug 23]
NYSDOH. Unpublished data; 2022.
Pawlowska M. Pegylated IFN-alpha-2a and ribavirin in the treatment of hepatitis C infection in children. Expert Opin Drug Saf 2015;14(3):343-48. [PMID: 25599750]
Pearlman B., Perrys M., Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol 2019;114(9):1550-52. [PMID: 31082871]
Poordad F., Felizarta F., Asatryan A., et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology 2017;66(2):389-97. [PMID: 28128852]
Prasad M. R., Honegger J. R. Hepatitis C virus in pregnancy. Am J Perinatol 2013;30(2):149-59. [PMID: 23389935]
Reddy K. R., Bourliere M., Sulkowski M., et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 2015;62(1):79-86. [PMID: 25846144]
Schillie S., Wester C., Osborne M., et al. CDC recommendations for hepatitis C screening among adults - United States, 2020. MMWR Recomm Rep 2020;69(2):1-17. [PMID: 32271723]
Simmons B., Saleem J., Heath K., et al. Long-term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis 2015;61(5):730-40. [PMID: 25987643]
Simmons B., Saleem J., Hill A., et al. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis 2016;62(6):683-94. [PMID: 26787172]
Sinclair S. M., Jones J. K., Miller R. K., et al. The Ribavirin Pregnancy Registry: an interim analysis of potential teratogenicity at the mid-point of enrollment. Drug Saf 2017;40(12):1205-18. [PMID: 28689333]
Smith-Palmer J., Cerri K., Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis 2015;15:19. [PMID: 25596623]
Sulkowski M. S., Chuang W. L., Kao J. H., et al. No evidence of reactivation of hepatitis B virus among patients treated with ledipasvir-sofosbuvir for hepatitis C virus infection. Clin Infect Dis 2016;63(9):1202-4. [PMID: 27486112]
Takayama H., Sato T., Ikeda F., et al. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol Res 2016;46(5):489-91. [PMID: 26297529]
Tasillo A., Eftekhari Yazdi G., Nolen S., et al. Short-term effects and long-term cost-effectiveness of universal hepatitis C testing in prenatal care. Obstet Gynecol 2019;133(2):289-300. [PMID: 30633134]
Terrault N. A., Zeuzem S., Di Bisceglie A. M., et al. Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology 2016;151(6):1131-40.e5. [PMID: 27565882]
Thomas D. L. Global control of hepatitis C: where challenge meets opportunity. Nat Med 2013;19(7):850-58. [PMID: 23836235]
UpToDate. Direct-acting antivirals for the treatment of hepatitis C virus infection. 2021 Apr 2. https://www.uptodate.com/contents/direct-acting-antivirals-for-the-treatment-of-hepatitis-c-virus-infection [accessed 2017 Jun 30]
van der Meer A. J., Veldt B. J., Feld J. J., et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama 2012;308(24):2584-93. [PMID: 23268517]
Vandenbulcke H., Moreno C., Colle I., et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: a prospective study. J Hepatol 2016;65(3):543-51. [PMID: 27180899]
Wang C., Ji D., Chen J., et al. Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol 2017;15(1):132-36. [PMID: 27392759]
Waruingi W., Mhanna M. J., Kumar D., et al. Hepatitis C virus universal screening versus risk based selective screening during pregnancy. J Neonatal Perinatal Med 2015;8(4):371-78. [PMID: 26836823]
Wijarnpreecha K., Thongprayoon C., Sanguankeo A., et al. Hepatitis C infection and intrahepatic cholestasis of pregnancy: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2017;41(1):39-45. [PMID: 27542514]
Wyles D., Weiland O., Yao B., et al. Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C virus infection. J Hepatol 2019;70(5):1019-23. [PMID: 30857780]
Yattoo G. N. Treatment of chronic hepatitis C with ledipasvir/sofosbuvir combination during pregnancy. Hepatol Int 2018;12(Suppl 2):S292-93.
Zeuzem S., Foster G. R., Wang S., et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018;378(4):354-69. [PMID: 29365309]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | July 31, 2017 |
| Date of current publication | April 17, 2023 |
| Highlights of changes, additions, and updates in the April 17, 2023 edition |
|
| Intended users | Clinicians in New York State who treat adults with chronic HCV |
| Lead author |
David E. Bernstein, MD |
| Writing group |
Joshua S. Aron, MD; Christine A. Kerr, MD; Colleen Flanigan, RN, MS; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD |
| Author and writing group conflict of interest disclosures | There are no author or writing group conflict of interest disclosures |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines | |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on February 8, 2024

