Purpose of This Guideline
Date of current publication: August 11, 2022
Lead author: Antonio E. Urbina, MD
Contributors: Nicole Bradley, PharmD, BCPS, BCIDP; Yuman Lee, PharmD, BCIDP, AAHIVP; John M. Conry, PharmD, AAHIVP, FNAP
Writing group: Steven M. Fine, MD, PhD; Rona Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Charles J. Gonzalez, MD; Christopher J. Hoffmann, MD, MPH
Committee: Medical Care Criteria Committee
Date of original publication: April 27, 2017
This guideline was developed by the New York State Department of Health AIDS Institute (NYSDOH AI) for primary care providers and other practitioners who are initiating therapy in nonpregnant, antiretroviral therapy (ART)-naive adults with HIV. The guideline aims to achieve the following goals:
- Provide a clear and concise roadmap for clinicians to follow in choosing from among several equally efficacious ART regimens based on individual patient characteristics and preferences.
- Provide a list of ART regimens to avoid.
- Provide dosing considerations for individuals with renal or hepatic impairment and important drug-drug and food interactions.
- Encourage clinicians to seek the assistance of an experienced HIV care provider when treating patients with extensive comorbidities.
- Integrate current evidence-based clinical recommendations into the healthcare-related implementation strategies of the New York State Ending the Epidemic initiative.
The NYSDOH AI is publishing this guideline at a critical time: 1) prompt initiation of ART is now recommended for all individuals diagnosed with HIV; 2) identifying and linking individuals with HIV to care and treatment that achieves optimal virologic suppression are crucial to the success of the New York State Ending the Epidemic initiative; and 3) the ability of primary care providers and other clinicians in New York State to properly select initial ART is key to the successful treatment of individuals with HIV.
Introduction: The NYSDOH AI Medical Care Criteria Committee recommendations for prescribing ART regimens for treatment-naive, nonpregnant adults (age ≥18 years) with HIV-1 and without acquired resistance are based on a comprehensive review of available clinical trial data. (For guidelines specific to treatment of adolescents with HIV, please consult the DHHS Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV). In formulating its recommendations for New York State, this committee balanced the strength of published evidence regarding efficacy of treatment regimens with factors that influence adherence, including pill burden, tolerability, and dosing schedule. Preferred regimens are supported by evidence and have favorable adherence profiles, with lower pill burdens, fewer adverse effects, and dosing schedules that may be easier for individuals to manage. Ranking of regimens in this manner is designed to inform discussion and decision-making with patients.
How to use this guideline: Tables presenting preferred and alternative regimens appear first (see guideline section Available ART Regimens). To help guide the choice among regimens of similar efficacy, each table includes comments that address selected pertinent issues regarding each regimen, such as limitations based on a patient’s kidney function and drug-drug interactions.
Other sections of the guideline include a review of relevant issues, patient considerations, essential laboratory assessments, and the rationale for the recommendations. Reference to the expanded information is crucial for addressing factors that may be of particular importance when individualizing a patient’s treatment, such as loss of bone mineral density with a regimen that includes tenofovir disoproxil fumarate and the conflicting data on cardiac risk with abacavir (see guideline section Specific Factors to Consider and Discuss With Patients). In addition, a review of psychosocial factors and individual patient preferences may help in the selection of an initial ART regimen. Importantly, a patient-centered approach that incorporates both clinical and nonclinical issues should be prioritized.
Scope: This guideline addresses initial treatment of HIV-1 infection with ART in nonpregnant adults.
- For information regarding ART in individuals who are or who may become pregnant, see DHHS Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States.
- For recommendations regarding the treatment of HIV-2 infection, see the NYSDOH AI guideline Diagnosis and Management of HIV-2 in Adults.
- For recommendations regarding second-line ART regiments, see the NYSDOH AI guideline Second-Line ART After Treatment Failure or for Regimen Simplification.
Note on “experienced” and “expert” HIV care providers: Throughout this guideline, when reference is made to “experienced HIV care provider” or “expert HIV care provider,” those terms are referring to the following 2017 NYSDOH AI definitions:
- Experienced HIV care provider: Practitioners who have been accorded HIV Experienced Provider status by the American Academy of HIV Medicine or have met the HIV Medicine Association’s definition of an experienced provider are eligible for designation as an HIV Experienced Provider in New York State. Nurse practitioners and licensed midwives who provide clinical care to individuals with HIV in collaboration with a physician may be considered HIV Experienced Providers as long as all other practice agreements are met (8 NYCRR 79-5:1; 10 NYCRR 85.36; 8 NYCRR 139-6900). Physician assistants who provide clinical care to individuals with HIV under the supervision of an HIV Specialist physician may also be considered HIV Experienced Providers (10 NYCRR 94.2)
- Expert HIV care provider: A provider with extensive experience in the management of complex patients with HIV.
Available ART Regimens
| Abbreviations used in this section: | |||
| Drug names: | Other: | ||
|
|
|
|
Note: The recommendations in this guideline pertain to initial ART regimens for adults with HIV who are not pregnant.
| RECOMMENDATIONS |
Regimen Selection
Expert Consultation
Follow-up
|
Note:
|
Available Antiretroviral Agents and Regimens
An initial ART regimen should be selected on the basis of patient preferences and clinical characteristics, and a preferred regimen should be used whenever possible. Each of the preferred, alternative, and other initial ART regimens for nonpregnant adults is expected to produce full viral suppression; however, the regimens differ in tolerability, possible toxicities, convenience, and the potential for drug-drug interactions, all of which can affect overall adherence and, therefore, suppression rates.
Preferred backbone: This committee recommends that either formulation of tenofovir (TAF or TDF) can be used as part of the backbone in preferred regimens. A recent meta-analysis of 14 clinical trials Pilkington, et al. 2020, comparing the efficacy and safety of TAF versus TDF in boosted and unboosted subgroups, found comparable efficacy and safety profiles with the use of unboosted TDF compared with TAF (see Table 1, below). Studies have shown that TDF-related renal toxicity is more common when using TDF in a regimen containing COBI or RTV Pilkington, et al. 2020; Hill, et al. 2018; Cuzin, et al. 2017; Ryom, et al. 2013; Goicoechea, et al. 2008. Therefore, this committee does not recommend TDF use with boosted regimens when initiating therapy.
In a study of ART-naive individuals, RAL HD 1200 mg once daily was noninferior to 400 mg tablets dosed twice daily and is thus preferred Cahn(b), et al. 2017.
Greater weight gain has also been observed after initiation of TAF or with a switch from TDF to TAF, especially in conjunction with INSTIs Łomiak, et al. 2021; Surial, et al. 2021; Bourgi(a), et al. 2020; Bourgi(b), et al. 2020; Calmy, et al. 2020; Lake, et al. 2020; Sax, et al. 2020; Venter, et al. 2020; Venter, et al. 2019. However, the predictors, mechanisms of actions, and clinical significance of these findings is unknown.
Third drug: An INSTI as the third drug is preferred over PIs and NNRTIs based on tolerability and a lower incidence of drug-drug interactions. RPV is not appropriate for patients with a viral load >100,000 copies/mL or CD4 count ≤200 cells/mm3 and is contraindicated with PPIs. TAF/FTC/RPV is included as an alternative regimen.
EFV-containing regimens, although efficacious, have been shown to be less well tolerated than the preferred or alternative regimens.
LPV/RTV-containing regimens are no longer included among the options for initial treatment because of pill burden and reduced tolerability in comparison with other boosted PIs.
When initiating ART at the time of HIV diagnosis (i.e., “rapid start” or “test and treat”), avoid regimens containing ABC unless the result of HLA-B*5701 testing is known to be negative.
Single-Tablet Regimens Versus Multi-Tablet Regimens
The advantages of STRs compared with MTRs include simplicity, convenience, and lower risk of selective nonadherence Gardner, et al. 2008. A recent meta-analysis demonstrated that STRs had better adherence rates when compared with MTRs of any frequency (once daily or twice daily) and had higher 48-week viral suppression rates with comparable adverse effects Clay, et al. 2015.
In another retrospective study, INSTI-based regimens generally had greater rates of suppression and a lower probability of viral rebound after suppression in comparison to NNRTI-based regimens, regardless of whether an STR or MTR was used, but STR INSTI-based therapy was more durable Mills(b), et al. 2016. In the same study, STR NNRTI-based therapy led to greater rates of suppression than MTR NNRTI-based therapy Mills(b), et al. 2016.
| Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; COBI, cobicistat; CrCl, creatinine clearance; DHHS, U.S. Department of Health and Human Services; FDA, U.S. Food and Drug Administration; FTC, emtricitabine; HBV, hepatitis B virus; INSTI, integrase strand transfer inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; RTV, ritonavir; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate.
Notes:
|
| Table 1: Important Clinical Considerations With Either TDF/FTC or TAF/FTC as Initial ART [a] |
If the patient is at risk of chronic kidney disease (e.g., age >40 years, with hypertension, diabetes, or preexisting mild kidney disease): The greater possibility of kidney disease among individuals who have risk factors is an essential component of the risk-benefit discussion and shared decision-making regarding initiation of tenofovir-containing regimens.
If the patient has osteopenia, osteomalacia, or osteoporosis:
If the patient has concerns about weight gain, hyperlipidemia, or metabolic disorders:
If the patient is an adolescent or youth:
If the patient is pregnant or attempting to conceive:
If the patient has active chronic HBV:
|
Other studies have demonstrated better efficacy and adherence, lower cost to patients, and fewer hospital admissions associated with STRs than with MTRs Griffith, et al. 2019; Mills(b), et al. 2016; Armstrong, et al. 2015; Maggiolo, et al. 2015; Hanna, et al. 2014; Nachega, et al. 2014; Sweet, et al. 2014; Cohen(a), et al. 2013; Colombo, et al. 2013; Raboud, et al. 2011; Bangalore, et al. 2007.
There are 3 STRs listed below as preferred regimens. It is possible that these regimens may contain 1 or more components that are not appropriate for an individual patient, do not allow for adjustment of individual components for renal function, have significant drug-drug interactions, are poorly tolerated, or may be more expensive than the individual components prescribed separately, particularly if available as generic formulations. With full adherence, any of the preferred or alternative regimens should lead to full suppression. This includes MTRs, which can be used when an STR is not possible or not tolerated. Cost and access may also be determinative factors.
For patients with impaired baseline renal function, separating the drugs into individual components and adjusting each may be appropriate (see guideline section ARV Dose Adjustments for Hepatic or Renal Impairment).
Table 2 includes initial ART regimens preferred by this committee; Table 3 lists alternative initial regimens. Table 4 lists other available ART regimens that this committee considers neither preferred nor alternative. Within each table, regimens are listed alphabetically. For specific details on choosing a regimen, see the discussions in other sections of this guideline and/or the package inserts for the drugs listed below.
| Abbreviations: ART, antiretroviral therapy; CrCl, creatinine clearance; DHHS, U.S. Department of Health and Human Services; NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; PEP, post-exposure prophylaxis.
Notes:
|
||
| Table 2: Preferred Initial Antiretroviral Therapy Regimens for Nonpregnant Adults [a] (listed alphabetically; for specific details, see guideline section Specific Factors to Consider and Discuss With Patients and drug package inserts) |
||
| Regimen | Comments | Rating |
| Available as a Single-Tablet Formulation | ||
| Abacavir/lamivudine/dolutegravir [b,c] (ABC/3TC/DTG; Triumeq) |
|
A1 |
| Lamivudine/dolutegravir [b,c] (3TC/DTG; Dovato) |
|
A1 |
| Tenofovir alafenamide/ emtricitabine/bictegravir [c] (TAF 25 mg/FTC/BIC; Biktarvy) |
|
A1 |
| Available as a Multi-Tablet Regimen With Once-Daily Dosing | ||
| Tenofovir alafenamide/ emtricitabine or tenofovir disoproxil fumarate/emtricitabine and dolutegravir [b,c] (TAF 25 mg/FTC or TDF 300 mg/FTC and DTG; Descovy or Truvada and Tivicay) |
|
A1 |
| Tenofovir alafenamide/ emtricitabine or tenofovir disoproxil fumarate/emtricitabine and raltegravir HD [c] (TAF 25 mg/FTC or TDF 300 mg/FTC and RAL HD; Descovy or Truvada and Isentress HD) |
|
A2 |
| Abbreviations: ART, antiretroviral therapy; CrCl, creatinine clearance; CYP, cytochrome P450; DHHS, U.S. Department of Health and Human Services.
Notes:
|
||
| Table 3: Alternative Initial ART Regimens for Nonpregnant Adults [a] (listed alphabetically; for specific details, see guideline section Specific Factors to Consider and Discuss With Patients and drug package inserts) |
||
| Regimen | Comments | Rating |
| Available as a Single-Tablet Formulation | ||
| Tenofovir alafenamide/ emtricitabine/darunavir/cobicistat [b] (TAF 10 mg/FTC/DRV/COBI; Symtuza) |
|
B2 |
| Tenofovir alafenamide/ emtricitabine/elvitegravir/cobicistat [b] (TAF 10 mg/FTC/EVG/COBI; Genvoya) |
|
B1 |
| Tenofovir alafenamide/ emtricitabine/rilpivirine [b] (TAF 25 mg/FTC/RPV; Odefsey) |
|
B3 |
| Tenofovir disoproxil fumarate/ lamivudine/doravirine [b] (TDF/3TC/DOR; Delstrigo) |
|
B1 |
| Available as a Multi-Tablet Regimen With Once-Daily Dosing | ||
| Abacavir/lamivudine and doravirine [b] (ABC/3TC and DOR; Epzicom and Pifeltro) Molina, et al. 2018 |
|
B2 |
| Tenofovir alafenamide/emtricitabine and doravirine [b] (TAF 25 mg/FTC and DOR; Descovy and Pifeltro) |
|
B2 |
| Available as a Multi-Tablet Regimen With Twice-Daily Dosing | ||
| Tenofovir alafenamide/emtricitabine or tenofovir disoproxil fumarate/emtricitabine and raltegravir [b] (TAF 25 mg/FTC or TDF 300 mg/FTC and RAL; Descovy or Truvada and Isentress) |
|
B3 |
Download Table 3: Alternative Initial ART Regimens for Nonpregnant Adults [a] Printable PDF
| Abbreviations: ART, antiretroviral therapy; CrCl, creatinine clearance; DHHS, U.S. Department of Health and Human Services; PPI, proton pump inhibitor.
Notes:
|
||
| Table 4: Other ART Regimens Not Included as Preferred or Alternative for Nonpregnant Adults [a] (listed alphabetically; for specific details, see guideline section Specific Factors to Consider and Discuss With Patients and drug package inserts) |
||
| Regimen | Comments | Rating |
| Available as a Single-Tablet Regimen | ||
| Tenofovir disoproxil fumarate/ emtricitabine/efavirenz [b] (TDF/FTC/EFV; Atripla) |
|
B1 |
| Tenofovir disoproxil fumarate/ emtricitabine/rilpivirine [b] (TDF/FTC/RPV; Complera) |
|
B1 |
| Available as a Multi-Tablet Regimen With Once-Daily Dosing | ||
| Abacavir/lamivudine and atazanavir and ritonavir [b,e] (ABC/3TC and ATV and RTV; Epzicom and Reyataz and Norvir) |
|
C1 |
| Abacavir/lamivudine and darunavir/cobicistat [b,e] (ABC/3TC and DRV/COBI; Epzicom and Prezcobix) |
|
B3 |
| Abacavir/lamivudine and darunavir and ritonavir [b,e] (ABC/3TC and DRV and RTV; Epzicom and Prezista and Norvir) |
|
B2 |
| Abacavir/lamivudine and efavirenz [b,e] (ABC/3TC and EFV; Epzicom and Sustiva) |
|
C1 |
| Tenofovir alafenamide/emtricitabine and efavirenz [b] (TAF 25 mg/FTC and EFV; Descovy and Sustiva) |
|
B3 |
| Abacavir/lamivudine and raltegravir HD [b,e] (ABC/3TC and RAL HD; Epzicom and Isentress HD) |
|
B3 |
| Available as a Multi-Tablet Regimen With Twice-Daily Dosing | ||
| Tenofovir disoproxil fumarate/emtricitabine and raltegravir [b] (TDF/FTC and RAL; Truvada and Isentress) |
|
B1 |
| Abacavir/lamivudine and raltegravir [b,e] (ABC/3TC and RAL; Epzicom and Isentress) |
|
B1 |
| USE OF DOLUTEGRAVIR IN INDIVIDUALS OF CHILDBEARING CAPACITY |
|
Reviewed and updated: Geoffrey A. Weinberg, MD, with the Medical Care Criteria Committee; May 2021 Evidence from multiple studies indicates no difference in rates of total birth defects among infants exposed to antiretroviral (ARV) medications during the first trimester compared with infants exposed later in pregnancy. ARVs are generally considered safe and may be taken by pregnant patients with HIV without increasing the risk of infant birth defects. The MCCC is providing the following updated information for medical care providers concerning preliminary reports that previously had linked dolutegravir (DTG) to neural tube defects (NTDs) in infants exposed to dolutegravir during the periconception period [Zash, et al. 2022]. Read the full statement. |
General Principles in Choosing an Initial ART Regimen
| Abbreviations used in this section: | |||
| Drug names: | Other: | ||
|
|
|
|
Goals of ART: The issue of when to start ART was settled with the publication of the START and TEMPRANO studies early in 2015 Danel, et al. 2015; Lundgren, et al. 2015. Treatment is now recommended for all individuals with confirmed HIV regardless of CD4 cell count or viral load.
The goal of ART is complete and durable suppression of plasma viremia while minimizing toxicity and maximizing quality of life. Properly selected ART may never require a change or adjustment once started. Treatment interruptions should be avoided El-Sadr, et al. 2006.
Since the approval of ZDV on March 19, 1987, there have been 30 individual agents approved for the treatment of HIV and 1 pharmacokinetic enhancer or “booster,” COBI, which is currently used to enhance the pharmacokinetics of EVG, ATV, or DRV. RTV at treatment doses is poorly tolerated and is used only at lower doses for pharmacokinetic boosting of PIs. An additional 18 FDA-approved FDCs are also available. These FDCs include STRs, of which there are 9 currently available that provide a complete and effective treatment regimen for HIV that is combined into 1 pill for use in properly selected individuals. The goal of initial therapy is to start a regimen that suits an individual’s lifestyle and is appropriate given existing baseline medical comorbidities (see Table 5, below).
Active drugs from 2 different classes: Although regimen options for treatment-naive, nonpregnant adults are constantly evolving, the same general principles that were established with the first effective and durable therapies are still true today Gulick, et al. 2000. The backbone of therapy has traditionally been 2 NRTIs paired with 1 of the following: an NNRTI, a boosted PI, or a boosted or unboosted INSTI. In a large meta-analysis, INSTIs were superior to other drug classes as a third drug Lee, et al. 2014, and DTG may have specific advantages because of the lack, to date, of documented resistance developing in ART-naive patients who initiate DTG-containing regimens Wainberg and Mesplede 2015. The entry inhibitors and fusion inhibitors are not recommended for initial therapy (see Table 6: Selected Drug-Drug Interactions to Discuss Before Initiating ART in Treatment-Naive Patients), but they may have a role in ART for treatment-experienced patients with extensive drug resistance (see All FDA-Approved HIV Medications, including generic and trade names).
The only 2-drug regimen that this committee recommends for ART initiation is DTG/3TC, although other dual- and even monotherapy regimens have been and continue to be studied Cahn, et al. 2020; Cahn, et al. 2019; Baril, et al. 2016; Cahn(a), et al. 2017; Maggiolo, et al. 2016; Bedimo, et al. 2014; Cahn, et al. 2014; Raffi, et al. 2014; Taiwo, et al. 2011. Importantly, use of DTG/3TC is recommended only after the result of HIV resistance and HBV testing are known. Further, this committee recommends against the use of DTG/3TC in patients with major NRTI resistance, including the M184V/I mutation.
If DTG/3TC is started in a patient with HBV infection, a third antiviral agent with activity against HBV should be added. Because the Gemini 1 and 2 studies restricted entry to patients with HIV-1 RNA ≤500,000 copies/mL, this committee recommends against use of DTG/3TC in patients with HIV-1 RNA >500,000 copies/mL.
TAF, a prodrug formulation for tenofovir, was developed as an alternative to TDF and has been approved as part of the following STRs:
- TAF 10 mg/FTC/COBI/EVG
- TAF 25 mg/FTC/RPV
- TAF 25 mg/FTC/BIC FDA(a) 2018; FDA(b) 2016; FDA(c) 2016
- TAF 10 mg/FTC/COBI/DRV FDA(b) 2018 and the FDC TAF 25 mg/FTC FDA(a) 2016
Oral administration of TAF results in lower circulating levels of tenofovir in plasma and affects markers of renal toxicity and bone mineral density less adversely than does TDF Mills(a), et al. 2016; Pozniak, et al. 2016; Sax, et al. 2015. Bioequivalence studies in healthy volunteers show that the TAF 10 mg dose administered with COBI 150 mg is equivalent to the TAF 25 mg dose without COBI Zack(a), et al. 2016; Zack(b), et al. 2016.
A switch study showed good maintenance of viral suppression when changing from TDF/FTC to TAF 10 mg/FTC if the third drug was a boosted PI, or to TAF 25 mg/FTC if the third drug was an unboosted NNRTI or INSTI Gallant, et al. 2016. Note that TAF 10 mg alone and TAF 10 mg/FTC are not currently FDA-approved.
Until further safety data are available, this committee has not included TAF 25 mg/FTC in combination with COBI or RTV as recommended regimens and recommends caution when using TAF 25 mg/FTC with regimens that contain either COBI or RTV in the setting of CrCl <50 mL/min.
COBI-boosted DRV was approved based on bioavailability studies FDA(d) 2016; Kakuda, et al. 2014. DRV/COBI has demonstrated comparable efficacy to RTV-boosted DRV in a single-arm study Tashima, et al. 2014. However, because randomized clinical trials that compare COBI- versus RTV-boosted DRV are not yet available, it has a lower strength of evidence rating. COBI-boosted ATV showed noninferiority when compared with RTV-boosted ATV with a TDF/FTC backbone in a randomized, double-blind study Gallant, et al. 2013.
| KEY POINT |
|
All of the currently recommended preferred regimens have similar virologic efficacy when measured by an “on-treatment” metric, but adherence, the potential for drug interactions, and tolerability under real-life conditions may inform the choice of preferred versus alternative versus other regimens.
The following general conclusions can be drawn based on currently available evidence from a number of pivotal studies:
- When ABC/3TC is used as a backbone with EFV or boosted ATV, time to failure was shorter in the ≥100,000 copies/mL viral load stratum when compared with a backbone of TDF/FTC Sax, et al. 2011; Post, et al. 2010; Sax, et al. 2009.
- DTG is as efficacious as (i.e., noninferior to) RAL Raffi, et al. 2013 and superior to both DRV/RTV Molina, et al. 2014 and coformulated TDF/FTC/EFV Walmsley, et al. 2015. DTG was superior at 48 weeks when combined with ABC/3TC as compared to TDF/FTC/EFV Walmsley, et al. 2013.
- RAL, although dosed twice daily, has a favorable tolerability profile, provides durable virologic control Lennox, et al. 2014; DeJesus, et al. 2012; Young, et al. 2010, and was superior to both DRV/RTV and ATV/RTV based on the cumulative incidence of virologic failure and tolerability Lennox, et al. 2014.
- In a study of ART-naive individuals, RAL HD 1200 mg once daily was noninferior to RAL 400 mg tablets dosed twice daily Cahn(b), et al. 2017.
- TAF/FTC/COBI/EVG as an STR was noninferior to the STR TDF/FTC/COBI/EVG, with fewer adverse effects on kidney function and bone mineral density Sax, et al. 2015.
- In 2 separate trials of treatment-naive individuals, TAF/FTC/BIC was noninferior to both TAF/FTC and DTG Sax, et al. 2017 and ABC/3TC/DTG Gallant, et al. 2017.
- RPV has equivalent efficacy relative to EFV when baseline viral load is <100,000 copies/mL and is better tolerated van Lunzen, et al. 2016; Behrens, et al. 2014; Cohen, et al. 2014; Cohen(b), et al. 2013; Cohen, et al. 2012. But RPV should not be initiated in individuals with baseline viral load >100,000 copies/mL or CD4 counts <200 cells/mm3.
- In 2 separate trials of treatment-naive individuals, TDF/3TC/DOR was noninferior to TDF/FTC/EFV, or DRV/RTV with either TDF/FTC or ABC/3TC Orkin, et al. 2019; Molina, et al. 2018.
- DRV/RTV once daily is better tolerated and noninferior to either ATV/RTV or LPV/RTV Lennox, et al. 2014; Orkin, et al. 2013, although LPV/RTV shows excellent efficacy when combined with either commonly used NRTI backbone Smith, et al. 2009 and when compared with ATV/RTV Molina, et al. 2008. One open-label study using ABC/3TC as the backbone combined with DRV/RTV showed good safety and efficacy Trottier, et al. 2012.
| Abbreviations: FDA, U.S. Food and Drug Administration; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor. | |
| Table 5: Individual Antiretroviral Medications or Combinations to Avoid in Initial Therapy for Nonpregnant Adults | |
| Antiretroviral Medication | Comments |
| Nevirapine (NVP; Viramune) | Life-threatening rash: Stevens-Johnson syndrome and toxic epidermal necrolysis are possible. |
|
Serious toxicities: Potentially fatal lactic acidosis, peripheral neuropathy, pancreatitis, lipoatrophy, and hepatic steatosis are possible. |
| Delavirdine (DLV; Rescriptor) | Thrice-daily dosing and inferior efficacy. |
| Etravirine (ETR; Intelence) | ETR does not have an FDA indication in ART-naive patients. |
|
Inferior efficacy and durability. |
| Zidovudine (ZDV; Retrovir) | Not well tolerated because of bone marrow suppression (notably anemia), headache, and myopathies. |
| Unboosted PIs | Inferior efficacy relative to boosted PIs. |
|
Either not well-studied or limited by dosing and side effects relative to recommended PIs. |
General Considerations With Initial ART Regimens
| Abbreviations used in this section: | |||
| Drug names: | Other: | ||
|
|
|
|
The recommended ART regimens should work well for the majority of patients, but some circumstances may make 1 regimen more favorable than another for a given individual. In general, an INSTI-based regimen will be the best option for most patients Mills(b), et al. 2016; Lee, et al. 2014. To date, no resistance has been reported in ART-naive patients treated with DTG as part of a registrational trial of combination therapy, suggesting that this medication may be an excellent choice, particularly given its tolerability and lack of drug-drug interactions Cevik, et al. 2020; Wainberg and Mesplede 2015. Regimens containing a boosted PI or DTG may be more appropriate when adherence is a concern, given the higher barrier to resistance. For patients with acute symptomatic infection or advanced HIV with an opportunistic infection, some experts would use both DTG and boosted DRV together with the NRTI backbone given the possibility of transmitted NRTI resistance until genotypic information is available, at which time the regimen can be adjusted. Consultation with an experienced HIV care provider is recommended when choosing a regimen for patients with extensive comorbidities, impaired renal function, HBV or HCV coinfection, or very high viral loads.
Early clinical trials in HIV used surrogate markers, such as viral load and CD4 cell count, or clinical endpoints, such as morbidity and mortality, to demonstrate superiority of new therapies over the “gold standard” treatment of the era. One of the trials that led to the 1996 approval of IDV compared IDV alone versus ZDV/3TC versus ZDV/3TC/IDV in ZDV- treatment-experienced patients, given that, at the time, dual NRTI treatment was considered acceptable Gulick, et al. 1997.
As treatment has evolved and become more effective, the use of clinical end points has become challenging; most trials in the current era of HIV therapy are powered to detect noninferiority when compared with standard of care. For a variety of reasons, including cost and complexity, it would be impractical to conduct head-to-head comparisons of all available regimens. Some STRs and FDCs have been approved primarily based on bioequivalence studies when compared with the individual components, such as TDF/FTC/EFV, ABC/3TC/DTG, TAF/FTC/RPV, TAF/FTC, and DRV/COBI.
Some of the cutoff values used for comparisons, such as viral load <100,000 copies/mL or CD4 count ≥200 cells/mm3, are somewhat arbitrary. For example, most studies including RPV show that its efficacy is diminished when initiated at viral loads ≥100,000 copies/mL, and 1 study showed that RPV worked less well than EFV-based therapy at a viral load of ≥500,000 copies/mL Domingo and Ribera 2013.
Some agents have been approved based on noninferiority to the relatively less well-tolerated TDF/FTC/EFV regimen, which is, nevertheless, a potent and effective regimen for those who tolerate it well. The higher prevalence of NNRTI resistance mutations when transmitted drug resistance occurs has prompted most experts to avoid NNRTI-based regimens if treatment is indicated before genotypic resistance testing results are available Panichsillapakit, et al. 2016; Rhee, et al. 2015; Stekler, et al. 2015. Although coformulated TAF/FTC/COBI/EVG is approved for use at any starting viral load, reports of failure using TDF/FTC/COBI/EVG, with resistance, have been documented in individuals with very high baseline viral loads >1,000,000 copies/mL Adams, et al. 2016; Rhee, et al. 2015.
A paucity of data is available demonstrating how different antiretroviral medications perform based on race and gender, although studies have suggested, for instance, that DRV/RTV is less well tolerated in women than in men and that discontinuation of DRV/RTV occurs at a higher rate among Black patients than among others Smith, et al. 2012; Currier, et al. 2010.
Long-acting injectable therapy: An injectable long-acting formulation of the INSTI CAB and the NNRTI RPV (CAB/RPV LA) has been approved by the FDA as replacement ART for adults and adolescents ≥12 years old who FDA 2021:
- Weigh ≥35 kg
- Do not have chronic hepatitis B virus infection
- Are virally suppressed (HIV-1 RNA level <50 copies/mL) on a stable ART regimen
- Have no history of treatment failure
- Have no known or suspected resistance to either CAB or RPV
Although not FDA-approved as initial ART or for individuals with detectable viremia, the use of CAB/RPV LA as replacement ART in virally suppressed patients engaged in care may be a suitable option for those who would prefer an alternative to daily oral therapy.
Specific Factors to Consider and Discuss With Patients
| Abbreviations used in this section: | |||
| Drug names: | Other: | ||
|
|
|
|
Before initiating ART, the following factors are important to consider and discuss with patients.
Age: As individuals with HIV age, they have a higher prevalence of comorbidities than younger patients with HIV and are likely to be on more non–HIV-specific medications, particularly cardiovascular or gastrointestinal agents, posing a higher risk for adverse interactions Marzolini, et al. 2011. For individuals older than 50 years, careful regimen selection, with the use of INSTIs when possible rather than cytochrome P450 inhibitors, such as COBI or RTV, can help minimize interactions. And use of TAF rather than TDF can lower the risk of renal and bone toxicity.
Comorbidities: Assessment for existing cardiovascular risk, renal disease or risk factors for the development of renal disease, hepatic disease, bone health, mental health, and substance use should be performed. Additionally, the risk for greater weight gain and potential exacerbation of metabolic complications with TAF- versus TDF-containing regimens, especially when combined with certain INSTI-based regimens, should be discussed prior to initiation of ART.
Cost: STRs may be favorable because of the lower copays that could be associated with fewer prescriptions. Conversely, the individual components of these regimens may be available generically as separate pills.
Dosing requirements (daily vs. twice daily): Most patients express a preference for once-daily dosing, especially if they are not taking other medications or are taking other medications that are dosed once daily. If individuals are already taking other medications that are dosed twice daily and report no adherence issues, twice-daily dosing is an acceptable option.
Drug-drug interactions: Because of some key drug-drug interactions, coadministration of some medications is to be avoided (see Table 6, below). For instance, PPIs should not be coadministered with oral RPV; however, injectable RPV can be used with PPIs. Given the availability of over-the-counter PPIs and the possibility that these drugs may be prescribed by a different care provider, this interaction is especially important to discuss with patients. In this case, to avoid unnecessary regimen changes once started, even patients who are not currently taking a PPIs should be asked whether they have needed PPIs in the past or may need them in the future. Dose limitations for metformin may also be required when combined with DTG and possibly BIC.
RTV and COBI have many significant and important interactions, including with cardiac medications. Methadone maintenance requirements may also change with some antiretroviral agents. A detailed review of all of a patient’s medications, including over-the-counter medications or supplements, is essential.
Before prescribing an ART regimen, using an automated interaction checker embedded in the electronic medical record or tools such as the following to check for potential drug-drug interactions with currently prescribed medications can help avoid serious problems:
| Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral medication; ATV, atazanavir; BIC, bictegravir; COBI, cobicistat; CrCl, creatinine clearance; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; PPI, proton pump inhibitor; RAL, raltegravir; RPV, rilpivirine; RTV, ritonavir.
Notes:
|
|
| Table 6: Selected Drug-Drug Interactions to Discuss Before Initiating ART in Treatment-Naive Patients | |
| Drugs | ARV(s): Comments |
| H2-blockers |
|
|
|
| Polyvalent cations [a] |
|
| PPIs |
|
| Metformin |
|
| Ethinyl estradiol and norethindrone [b] |
|
| Factor Xa inhibitors |
|
| Platelet inhibitors |
|
Food requirements: Because an individual may have a strong preference for taking medication with or without food, it is important to discuss which medications must be taken on an empty stomach, which must be taken with food, and which can be taken with or without food, as listed in Box 1, below.
| Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; BIC, bictegravir; COBI, cobicistat; DOR, doravirine; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; RAL, raltegravir; RPV, rilpivirine; RTV, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. | ||
| Box 1: Antiretroviral Medications That Can be Taken With or Without Food, Must Be Taken With Food, or Must Be Taken on an Empty Stomach | ||
| Take With or Without Food |
Take With Food | Take on Empty Stomach |
|
|
|
Known adverse effects and toxicities: Review known and potential adverse effects in advance.
Number of pills: Some patients feel strongly that the fewer the number of pills, the better. For others, the greatest concern may be the ability to take all pills (regardless of the number) together once daily. Sometimes using individual agents rather than a multi-agent FDC or STR may be attractive depending on pill size. In rare cases, individuals who either cannot or will not swallow pills may need liquid formulations or pill crushing. Table 7, below, presents an abbreviated summary of commonly used ARVs and their availability in liquid formulation and/or the acceptability of crushing or dissolving them prior to ingestion.
| Table 7: Acceptable Alternative Formulations and Methods of Administration of Antiretroviral Medications | ||
| Drug | Available as Liquid, Powder, or Chewable Tablet? | Can Tablet Be Split/Crushed/Dissolved? |
| Single-Tablet Formulations | ||
| Abacavir/lamivudine/dolutegravir (ABC/3TC/DTG; Triumeq) |
No | Probably acceptable to split/crush |
| Tenofovir alafenamide/ emtricitabine/bictegravir (TAF/FTC/BIC; Biktarvy) |
No | No data; not recommended |
| Tenofovir alafenamide/ emtricitabine/cobicistat/darunavir (TAF/FTC/COBI/DRV; Symtuza) |
No | No data; not recommended |
| Tenofovir alafenamide/ emtricitabine/elvitegravir/cobicistat (TAF/FTC/COBI/EVG; Genvoya) |
No | No data; not recommended |
| Tenofovir alafenamide/ emtricitabine/rilpivirine (TAF/FTC/RPV; Odefsey) |
No | No data; not recommended |
| Tenofovir disoproxil fumarate/ lamivudine/doravirine (TDF/3TC/DOR); Delstrigo) |
No | No data; not recommended |
| Tenofovir disoproxil fumarate/ emtricitabine/efavirenz (TDF/FTC/EFV; Atripla) |
No | No data; not recommended |
| Dolutegravir/lamivudine (DTG/3TC; Dovato) |
No | No data; not recommended |
| Tenofovir disoproxil fumarate/ emtricitabine/rilpivirine (TDF/FTC/RPV; Complera) |
No | No data; not recommended |
| Fixed-Dose Combinations | ||
| Abacavir/lamivudine (ABC/3TC; Epzicom) |
See individual components below | Probably acceptable to split/crush |
| Darunavir/cobicistat (DRV/COBI; Prezcobix) |
No | No |
| Tenofovir alafenamide/emtricitabine (TAF/FTC; Descovy) |
No | No |
| Tenofovir disoproxil fumarate/ emtricitabine (TDF/FTC; Truvada) |
See individual components below | Acceptable to crush/dissolve |
| Zidovudine/lamivudine (ZDV/3TC; Combivir) |
See individual components below | Probably acceptable to split/crush |
| Individual Drugs | ||
| Abacavir (ABC; Ziagen) |
Oral solution (20 mg/mL) | No data |
| Atazanavir (ATV; Reyataz) |
Oral dispersible powder (50 mg/packet) | Can open capsule and sprinkle contents |
| Darunavir (DRV; Prezista) |
Oral suspension (100 mg/mL) | Probably acceptable to crush |
| Doravirine (DOR; Pifeltro) |
No | No data |
| Dolutegravir (DTG; Tivicay) |
No | Acceptable to crush |
| Efavirenz (EFV; Sustiva) |
No | No |
| Elvitegravir (EVG; Vitekta) |
No | No data |
| Emtricitabine (FTC; Emtriva) |
Oral solution (10 mg/mL) | Acceptable to open and dissolve in water |
| Lamivudine (3TC; Epivir) |
Oral solution (10 mg/mL) | Acceptable to crush or split |
| Raltegravir (RAL; Isentress) |
Chewable tablet (25 mg, 100 mg); oral powder for suspension (100 mg/packet); neither is bioequivalent to the 400 mg adult dose | Not recommended |
| Raltegravir HD (RAL HD; Isentress HD) |
No | No data, not recommended |
| Rilpivirine (RPV; Edurant) |
No | No data, not recommended |
| Ritonavir (RTV; Norvir) |
Oral solution (80 mg/mL) | No |
| Tenofovir alafenamide (TAF; Vemlidy) | No | Acceptable to crush |
| Tenofovir disoproxil fumarate (TDF; Viread) |
Oral powder mixed with soft food only (40 mg/1 g) | Acceptable to dissolve in water |
Pill size: Use images or real examples to give patients an idea of pill size before they fill the prescription. TAF/FTC/BIC and TAF/FTC/RPV are the smallest STR pills.
Pregnancy or conception planning: Individuals of childbearing potential should receive a pregnancy test and be assessed for use of contraception. When selecting an initial regimen for those who are not using effective contraception or who are contemplating pregnancy, clinicians should consult DHHS Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States.
All patients should be assessed for conception plans, which also provides an opportunity to discuss PrEP for partners without HIV. Additionally, all individuals with HIV should be informed that maintaining a plasma HIV RNA level <200 copies/mL with ART prevents sexual transmission of HIV to their partners.
Special Considerations for Comorbid Conditions
| Abbreviations used in this section: | |||
| Drug names: | Other: | ||
|
|
|
|
Bone disease: TDF causes a decrease in bone mineral density in all patients after initiation of ART and should be used with caution in patients with preexisting severe osteoporosis McComsey, et al. 2011; Stellbrink, et al. 2010; Perrot, et al. 2009. Some experts recommend baseline bone densitometry screening for osteoporosis in postmenopausal women and in men and transgender women older than 50 years who have HIV Aberg, et al. 2014. The TAF formulation available currently in TAF/FTC, TAF/FTC/EVG/COBI, TAF/FTC/BIC, and TAF/FTC/RPV is an alternative, with lower markers of bone turnover in clinical trials Bonora, et al. 2016; Pozniak, et al. 2016.
Cardiovascular risks: COBI- or RTV-containing regimens typically elevate lipids; TAF and certain INSTIs can cause greater weight gain than other ART regimens, with the potential for increased risk of metabolic complications Łomiak, et al. 2021; Surial, et al. 2021; Bourgi(a), et al. 2020; Bourgi(b), et al. 2020; Calmy, et al. 2020; Lake, et al. 2020; Sax, et al. 2020; Venter, et al. 2020; Venter, et al. 2019. TDF-containing regimens can have a beneficial effect on lipids Souza, et al. 2013. ABC has been associated with a higher risk of myocardial infarction in some studies Marcus, et al. 2016; Choi, et al. 2011; Obel, et al. 2010; Sabin, et al. 2008; SMART/INSIGHT and D:A:D Study Groups 2008, whereas other studies have not confirmed this association Ding, et al. 2012; Bedimo, et al. 2011; Ribaudo, et al. 2011; Brothers, et al. 2009. Based on the available data, ABC should be used with caution in patients with multiple cardiac risk factors or known coronary heart disease; however, the absolute risk of myocardial infarction remains low, and no clear causality has been established. In the appropriate clinical circumstance, such as for a patient with impaired renal function, the use of ABC would be acceptable Llibre and Hill 2016. Clinicians should be made aware of the conflicting study data and share this information with patients.
Liver disease: In patients with existing liver disease of any etiology, dose adjustment of ARVs may be required depending on the severity of hepatic impairment (see guideline section ARV Dose Adjustments for Hepatic or Renal Impairment).
Mental health and substance use: Factors that may influence adherence should be addressed. There are also potential interactions between illicit (e.g., methamphetamine) and licit substances (e.g., methadone) and ARVs Kumar, et al. 2015.
| KEY POINT |
|
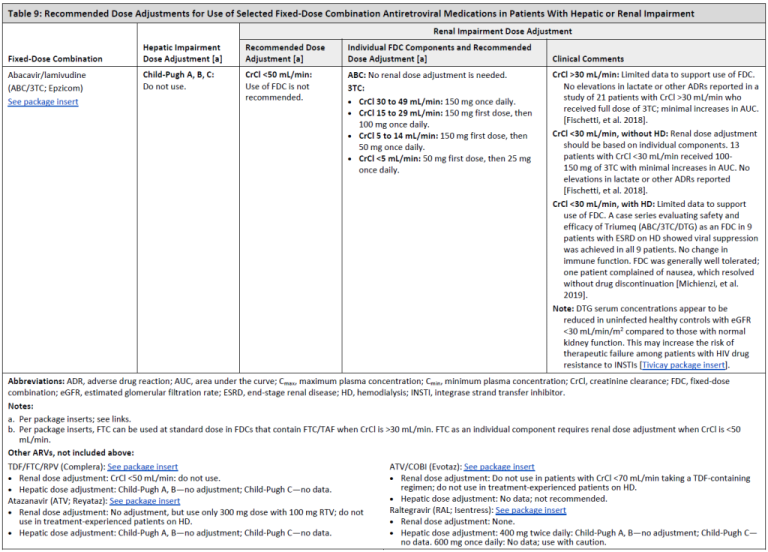
Renal function: TDF can cause renal tubular dysfunction, such as acquired Fanconi syndrome Zimmermann, et al. 2006; Karras, et al. 2003. The risk of renal impairment has been shown to be elevated in patients with preexisting renal disease, longer treatment duration, low body weight, and when used in conjunction with RTV- or COBI-boosted regimens Mocroft, et al. 2016; Gervasoni, et al. 2013. In general, full-dose TDF should be used with caution in patients with baseline CrCl <70 mL/min and should be adjusted or changed to an alternative agent if CrCl decreases to <50 mL/min; TAF is a better choice in these patients. As noted above, TAF 25 mg/FTC should be used with caution in boosted regimens when CrCl is <50 mL/min.
Both ATV/RTV and LPV/RTV have also been independently associated with a greater decrease in renal function over time than NNRTI-based regimens Quesada, et al. 2015; Goicoechea, et al. 2008. COBI, and to a lesser extent DTG, can inhibit the excretion of creatinine, with expected elevations of creatinine at initiation of therapy. However, such increases are not clinically relevant and do not significantly affect glomerular filtration rate Lepist, et al. 2014; Koteff, et al. 2013; German(a), et al. 2012.
Although DTG is highly bound to plasma proteins and is unlikely to be removed by dialysis, it has not been studied in this population FDA 2013; therefore, RAL or a boosted PI with renally adjusted 3TC and either ABC or once-weekly TDF are usually the regimens of choice in this population.
Additional information on prescribing agents for patients with reduced renal function is available in the guideline section ARV Dose Adjustments for Hepatic or Renal Impairment.
Very high viral load (HIV RNA level >750,000 copies/mL): In some cases, experts will recommend use of both boosted DRV and DTG in addition to 2 NRTIs when a patient’s viral load is very high, with possible simplification once viral suppression is achieved. Numerous switch studies have demonstrated the safety of simplifying ARV regimens in virally suppressed individuals with no preexisting drug resistance Cazanave, et al. 2015; Arribas, et al. 2014; Mills, et al. 2013; Fisher, et al. 2009. Consultation with an experienced HIV care provider in these situations is helpful.
| KEY POINTS |
|
ART-Initiation Laboratory Testing
| Abbreviations used in this section: | |||
| Drug names: | Other: | ||
|
|
|
|
Baseline CD4 cell count: Some regimens should not be used when the CD4 count is <200 cells/mm3 because of an increased risk of treatment failure (see Table 8, below). When Pneumocystis jiroveci pneumonia prophylaxis is indicated, it may be prudent to defer ART for 7 to 10 days if 2 medications that may cause rash will be started, such as TMP-SMX and EFV.
Baseline HIV genotypic resistance profile: Genotypic resistance testing that includes the protease, reverse transcriptase, and integrase genes should be obtained at diagnosis (or initial visit if not done previously), but ART initiation should not be delayed pending the results Kuritzkes, et al. 2008; Borroto-Esoda, et al. 2007.
Transmitted integrase resistance was identified in 0.7% of genotypic resistance tests obtained within 3 months of HIV diagnosis from 2013 to2017 in New York State Wang, et al. 2019. Similarly, 0.8% of baseline genotypic resistance tests across 23 U.S. jurisdictions of the CDC reported INSTI resistance, which had a higher prevalence (1.6%) in metropolitan areas (population 50,000 to 500,000) McClung, et al. 2022. Although INSTI resistance overall remains rare, most experts believe that transmission of INSTI resistance will increase over time, given that this class of ARV has become the preferred therapy for ART initiation (including rapid ART initiation) in all major guidelines.
Consultation with a care provider experienced in ART management is warranted when patients have baseline resistance that requires treatment with a regimen other than the listed preferred or alternative regimens. If treatment is indicated before genotypic resistance testing results are available, NNRTI-based regimens should be avoided because of the higher prevalence of transmitted resistance in NNRTIs than in PIs or INSTIs Panichsillapakit, et al. 2016; Rhee, et al. 2015; Stekler, et al. 2015. In the case of, for example, a patient with symptomatic acute HIV or advanced HIV with an opportunistic infection, some experts would include a second-generation INSTI (DTG or BIC or boosted DRV, or both together) with the NRTI backbone, given the possibility of transmitted NRTI resistance, with possible simplification once genotypic information is available.
Baseline viral load: Some regimens should not be used when the HIV RNA level is ≥100,000 copies/mL (see Table 8, below, and comments in the tables of preferred and alternative ART regimens).
Coinfections: Patients should be assessed for chronic HBV, HCV coinfection, and TB. The ART regimen for individuals with chronic HBV should treat both HIV and HBV when possible. For those planning concurrent HCV treatment or treatment for active TB, drug-drug interactions will play an important role in the selection of a regimen. The University of Liverpool HEP Drug Interactions Checker is a useful resource for identifying drug-drug interactions.
Creatinine clearance level: Some ARVs are contraindicated below a given CrCl level, and some may need adjustments that require the use of individual elements of an FDC or STR rather than the single-tablet version of the drug (see guideline section ARV Dose Adjustments for Hepatic or Renal Impairment).
Hepatic profile: Some ARVs require dose adjustment in the presence of impaired liver function; patients with abnormal liver enzyme levels or evidence of decreased synthetic function should be assessed for underlying liver disease (see guideline sections Special Considerations for Comorbid Conditions and ARV Dose Adjustments for Hepatic or Renal Impairment).
| KEY POINTS |
|
HLA-B*5701 testing: To avoid potentially serious or life-threatening hypersensitivity reactions, HLA-B*5701 testing is mandatory before initiating ART that includes ABC Mallal, et al. 2008; Saag, et al. 2008.
Initiation of the regimens listed in Table 8, below, is contraindicated based on the listed baseline laboratory parameters.
| Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV, atazanavir; BIC, bictegravir; COBI, cobicistat; CrCl, creatinine clearance; DOR, doravirine; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; RAL, raltegravir; RPV, rilpivirine; RTV, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Notes:
|
|
| Table 8: ART Regimens That Are Not Recommended Based on Routine Baseline Laboratory Parameters [a] | |
| Laboratory Parameter | ART Regimens That Are Not Recommended |
| HIV RNA level ≥100,000 copies/mL |
|
| CD4 count <200 cells/mm3 |
|
| CrCl <50 mL/min |
|
| CrCl <30 mL/min |
|
ARV Dose Adjustments for Hepatic or Renal Impairment
Recommended Dose Adjustments for Use of Selected Fixed-Dose Combination Antiretroviral Medications in Patients With Hepatic or Renal Impairment
Table 9, Page 1
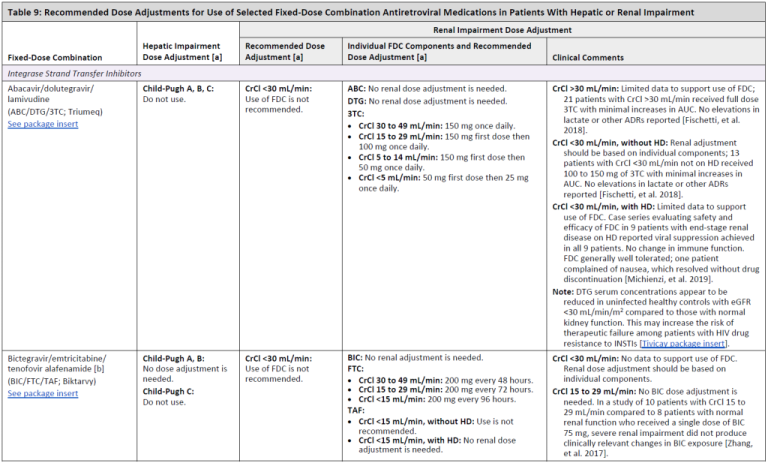
Table 9, Page 2
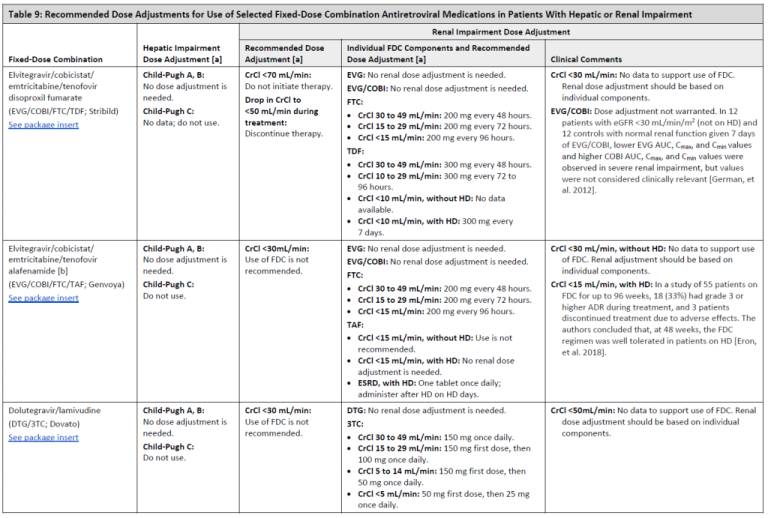
Table 9, Page 3
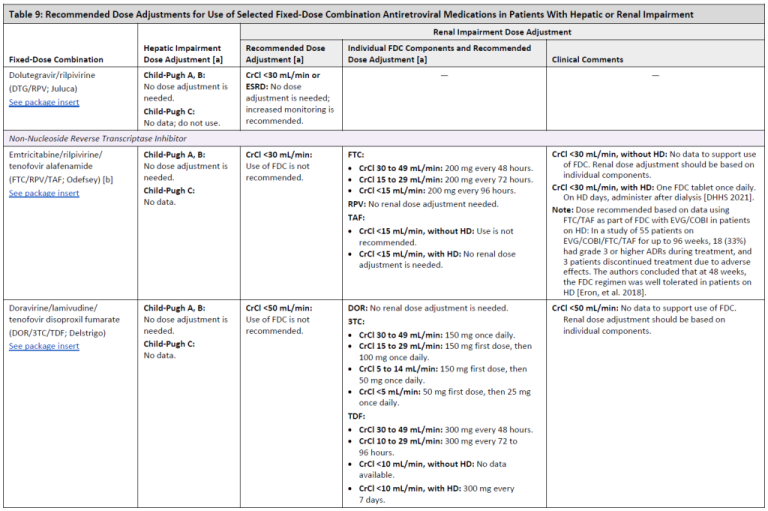
Table 9, Page 4
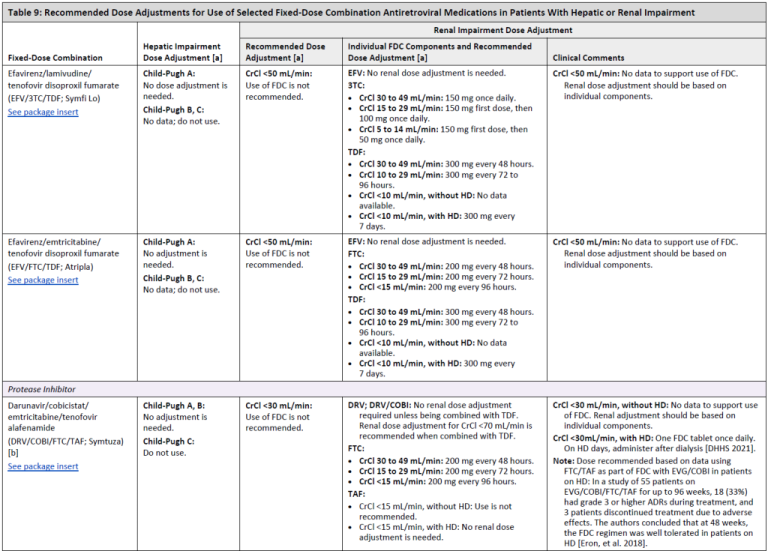
Table 9, Page 5
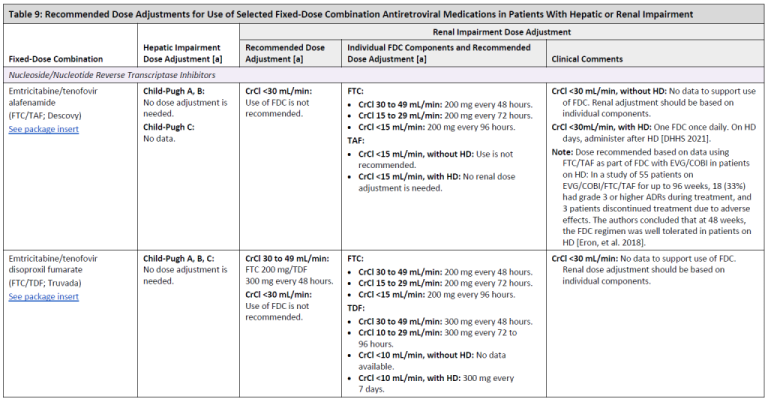
Table 9, Page 6
DRUG MANUFACTURER PACKAGE INSERTS
Atripla: FDA. Atripla (efavirenz/emtricitabine/tenofovir disoproxil fumarate) tablets for oral use. 2006. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021937s037lbl.pdf [accessed 2020 Mar 5].
Biktarvy: FDA. Biktarvy (bictegravir, emtricitabine, and tenofovir alafenamide) tablets, for oral use. 2018 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210251s000lbl.pdf [accessed 2020 Mar 5].
Complera: FDA. Complera (emtricitabine/rilpivirine/tenofovir disoproxil fumarate) tablets, for oral use. 2013 Jan. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202123s003lbl.pdf [accessed 2020 May 14].
Descovy: FDA. Descovy (emtricitabine and tenofovir alafenamide) tablets, for oral use. 2016 Apr. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208215s000lbl.pdf [accessed 2020 Mar 5].
Delstrigo: FDA. Delstrigo (doravirine, lamivudine, and tenofovir disoproxil fumarate) tablets, for oral use. 2018 Aug. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210807s000lbl.pdf [accessed 2020 Mar 5].
Dovato: FDA. Dovato (dolutegravir and lamivudine) tablets, for oral use. 2019 Apr. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211994s000lbl.pdf [accessed 2020 Mar 5].
Epzicom: FDA. Epzicom (abacavir sulfate and lamivudine) tablets for oral use. 2012 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021652s015lbl.pdf [accessed 2020 Mar 5].
Evotaz: FDA. Evotaz (atazanavir and cobicistat) tablets, for oral use. 2015 Jan. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206353s000lbl.pdf [accessed 2020 May 14].
Genvoya: FDA. Genvoya (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets, for oral use. 2015 Nov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207561s000lbl.pdf [accessed 2020 Mar 5].
Isentress: FDA. Isentress (raltegravir) tablets for oral use. 2013 June. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022145s029lbl.pdf [accessed 2020 May 14].
Juluca: FDA. Juluca (dolutegravir and rilpivirine) tablets, for oral use. 2017 Nov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/210192s000lbl.pdf [accessed 2020 Mar 5].
Odefsey: FDA. Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide) tablets, for oral use. 2016 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208351s000lbl.pdf [accessed 2020 Mar 5].
Reyataz: FDA. Reyataz (atazanavir) capsules, for oral use. 2016 Sept. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021567s039,206352s004lbl.pdf [accessed 2020 May 14].
Stribild: FDA. Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) tablets, for oral use. 2016 Sep. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203100s024lbl.pdf [accessed 2020 Mar 5].
Symfi Lo: FDA. Symfi lo (efavirenz, lamivudine, and tenofovir disoproxil fumarate) tablets, for oral use. 2018 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208255s000lbl.pdf [accessed 2020 Mar 5].
Symtuza: FDA. Symtuza (darunavir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets, for oral use. 2018 Jul. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210455s000lbl.pdf [accessed 2020 Mar 5].
Triumeq: FDA. Triumeq (abacavir, dolutegravir, and lamivudine) tablets, for oral use. 2017 Nov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205551s011lbl.pdf [accessed 2020 Mar 5].
Truvada: FDA. Truvada (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use. 2004. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021752s047lbl.pdf [accessed 2020 Mar 5].
Tivicay: FDA. Tivicay (dolutegravir) tablets, for oral use. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf [accessed 2020 Apr 13].
All Recommendations
| ALL RECOMMENDATIONS: SELECTING AN INITIAL ART REGIMEN |
Regimen Selection
Expert Consultation
Follow-up
|
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; DTG, dolutegravir; HBV, hepatitis B virus. Note:
|
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
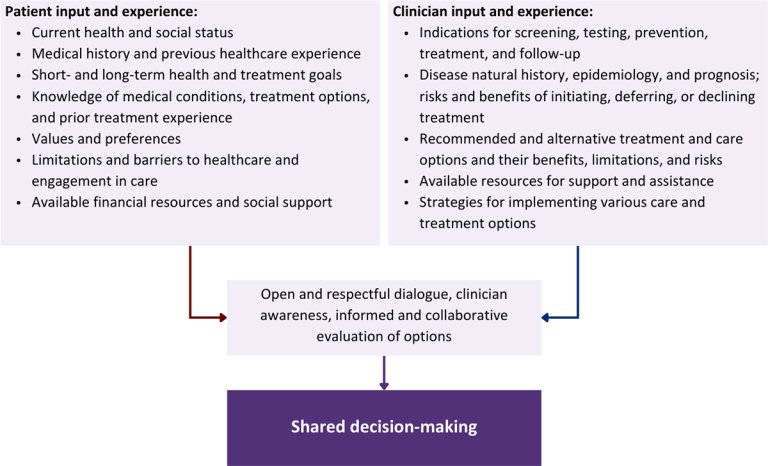
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Aberg J. A., Gallant J. E., Ghanem K. G., et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58(1):1-10. [PMID: 24343580]
Adams J. L., Byrne D., Pepe R., et al. Virological failure in two patients with HIV-1 RNA viral loads >1,000,000 copies/ml initiated on elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate. Antivir Ther 2016;21(2):175-80. [PMID: 26308882]
Armstrong B., Chan D. J., Stewart M. J., et al. Single tablet regimen usage and efficacy in the treatment of HIV infection in Australia. AIDS Res Treat 2015;2015:570316. [PMID: 26550490]
Arribas J. R., Pialoux G., Gathe J., et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis 2014;14(7):581-89. [PMID: 24908551]
Bangalore S., Kamalakkannan G., Parkar S., et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120(8):713-19. [PMID: 17679131]
Baril J. G., Angel J. B., Gill M. J., et al. Dual therapy treatment strategies for the management of patients infected with HIV: A systematic review of current evidence in ARV-naive or ARV-experienced, virologically suppressed patients. PLoS One 2016;11(2):e0148231. [PMID: 26849060]
Bedimo R. J., Drechsler H., Jain M., et al. The RADAR study: week 48 safety and efficacy of RAltegravir combined with boosted DARunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. Impact on bone health. PLoS One 2014;9(8):e106221. [PMID: 25170938]
Bedimo R. J., Westfall A. O., Drechsler H., et al. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis 2011;53(1):84-91. [PMID: 21653308]
Behrens G., Rijnders B., Nelson M., et al. Rilpivirine versus efavirenz with emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected patients with HIV-1 RNA </=100,000 copies/mL: week 96 pooled ECHO/THRIVE subanalysis. AIDS Patient Care STDS 2014;28(4):168-75. [PMID: 24660840]
Bonora S., Calcagno A., Trentalange A., et al. Elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide for the treatment of HIV in adults. Expert Opin Pharmacother 2016;17(3):409-19. [PMID: 26642079]
Borroto-Esoda K., Waters J. M., Bae A. S., et al. Baseline genotype as a predictor of virological failure to emtricitabine or stavudine in combination with didanosine and efavirenz. AIDS Res Hum Retroviruses 2007;23(8):988-95. [PMID: 17725415]
Bourgi(a) K., Rebeiro P. F., Turner M., et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020;70(7):1267-74. [PMID: 31100116]
Bourgi(b) K., Jenkins C. A., Rebeiro P. F., et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc 2020;23(4):e25484. [PMID: 32294337]
Brothers C. H., Hernandez J. E., Cutrell A. G., et al. Risk of myocardial infarction and abacavir therapy: no increased risk across 52 GlaxoSmithKline-sponsored clinical trials in adult subjects. J Acquir Immune Defic Syndr 2009;51(1):20-28. [PMID: 19282778]
Cahn P., Andrade-Villanueva J., Arribas J. R., et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis 2014;14(7):572-80. [PMID: 24783988]
Cahn P., Madero J. S., Arribas J. R., et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019;393(10167):143-55. [PMID: 30420123]
Cahn P., Madero J. S., Arribas J. R., et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr 2020;83(3):310-18. [PMID: 31834000]
Cahn(a) P., Rolón M. J., Figueroa M. I., et al. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc 2017;20(1):21678. [PMID: 28537061]
Cahn(b) P., Kaplan R., Sax P. E., et al. Raltegravir 1200 mg once daily versus raltegravir 400 mg twice daily, with tenofovir disoproxil fumarate and emtricitabine, for previously untreated HIV-1 infection: a randomised, double-blind, parallel-group, phase 3, non-inferiority trial. Lancet HIV 2017;4(11):e486-94. [PMID: 28918877]
Calmy A., Tovar Sanchez T., Kouanfack C., et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV 2020;7(10):e677-87. [PMID: 33010241]
Cevik M., Orkin C., Sax P. E. Emergent resistance to dolutegravir among INSTI-naive patients on first-line or second-line antiretroviral therapy: a review of published cases. Open Forum Infect Dis 2020;7(6):ofaa202. [PMID: 32587877]
Choi A. I., Vittinghoff E., Deeks S. G., et al. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS 2011;25(10):1289-98. [PMID: 21516027]
Clay P. G., Nag S., Graham C. M., et al. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine (Baltimore) 2015;94(42):e1677. [PMID: 26496277]
Cohen C., Wohl D., Arribas J. R., et al. Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected adults. Aids 2014;28(7):989-97. [PMID: 24508782]
Cohen C. J., Molina J. M., Cahn P., et al. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naive HIV-1-infected patients: pooled results from the phase 3 double-blind randomized ECHO and THRIVE trials. J Acquir Immune Defic Syndr 2012;60(1):33-42. [PMID: 22343174]
Cohen(a) C. J., Meyers J. L., Davis K. L. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US Medicaid population with HIV. BMJ Open 2013;3(8):e003028. [PMID: 23906955]
Cohen(b) C. J., Molina J. M., Cassetti I., et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two phase III randomized trials. AIDS 2013;27(6):939-50. [PMID: 23211772]
Colombo G. L., Di Matteo S., Maggiolo F. Antiretroviral therapy in HIV-infected patients: a proposal to assess the economic value of the single-tablet regimen. Clinicoecon Outcomes Res 2013;5:59-68. [PMID: 23430273]
Currier J., Averitt Bridge D., Hagins D., et al. Sex-based outcomes of darunavir-ritonavir therapy: a single-group trial. Ann Intern Med 2010;153(6):349-57. [PMID: 20855799]
Cuzin L., Pugliese P., Allavena C., et al. Antiretroviral therapy as a risk factor for chronic kidney disease: results from traditional regression modeling and causal approach in a large observational study. PLoS One 2017;12(12):e0187517. [PMID: 29216208]
Danel C., Moh R., Gabillard D., et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373(9):808-22. [PMID: 26193126]
DeJesus E., Rockstroh J. K., Lennox J. L., et al. Efficacy of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: week-192 overall and subgroup analyses from STARTMRK. HIV Clin Trials 2012;13(4):228-32. [PMID: 22849964]
DHHS(a). Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2021 Aug 16. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines [accessed 2018 May 1]
DHHS(b). Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States. 2021 Feb 10. https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new-guidelines [accessed 2018 May 1]
Ding X., Andraca-Carrera E., Cooper C., et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr 2012;61(4):441-47. [PMID: 22932321]
Domingo P., Ribera E. [Data on rilpivirine in treatment-naive patients. Lessons from ECHO, THRIVE and STaR]. Enferm Infecc Microbiol Clin 2013;31(Suppl 2):20-29. [PMID: 24252530]
El-Sadr W. M., Lundgren J. D., Neaton J. D., et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355(22):2283-96. [PMID: 17135583]
Eron(a) J. J., Orkin C., Gallant J., et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS 2018;32(11):1431-42. [PMID: 29683855]
Eron(b) J. J., Lelievre J. D., Kalayjian R., et al. Safety of elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in HIV-1-infected adults with end-stage renal disease on chronic haemodialysis: an open-label, single-arm, multicentre, phase 3b trial. Lancet HIV 2018;6(1):e15-24. [PMID: 30555051]
FDA. Tivicay (dolutegravir) tablets for oral use. 2013 Aug. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf [accessed 2018 May 2]
FDA. Cabenuva (cabotegravir extended-release injectable suspension; rilpivirine extended-release injectable suspension), co-packaged for intramuscular use. 2021 Jan. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212888s000lbl.pdf [accessed 2022 June 6]
FDA(a). Descovy (emtricitabine and tenofovir alafenamide) tablets, for oral use. 2016 Apr. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208215s000lbl.pdf [accessed 2018 May 2]
FDA(a). Biktarvy (bictegravir, emtricitabine, and tenofovir alafenamide) tablets, for oral use. 2018 Feb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210251s000lbl.pdf [accessed 2018 May 2]
FDA(b). Genvoya (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets, for oral use. 2016 Dec. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207561s002lbl.pdf [accessed 2018 May 2]
FDA(b). Symtuza (darunavir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets, for oral use. 2018 Jul. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210455s000lbl.pdf [accessed 2018 Oct 30]
FDA(c). Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide) tablets, for oral use. 2016 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208351s000lbl.pdf [accessed 2018 May 2]
FDA(d). Prezcobix (darunavir and cobicistat) tablets, for oral use. 2016 Mar. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205395s001lbl.pdf [accessed 2018 May 2]
Fischetti B., Shah K., Taft D. R., et al. Real-world experience with higher-than-recommended doses of lamivudine in patients with varying degrees of renal impairment. Open Forum Infect Dis 2018;5(10):ofy225. [PMID: 30302352]
Fisher M., Moyle G. J., Shahmanesh M., et al. A randomized comparative trial of continued zidovudine/lamivudine or replacement with tenofovir disoproxil fumarate/emtricitabine in efavirenz-treated HIV-1-infected individuals. J Acquir Immune Defic Syndr 2009;51(5):562-68. [PMID: 19561519]
Gallant J., Daar E. S., Raffi F., et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV 2016;3(4):e158-65. [PMID: 27036991]
Gallant J., Koenig E., Andrade-Villanueva J., et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: week 48 results. J Infect Dis 2013;208(1):32-39. [PMID: 23532097]
Gallant J., Lazzarin A., Mills A., et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017;390(10107):2063-72. [PMID: 28867497]
Gardner E. M., Sharma S., Peng G., et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS 2008;22(1):75-82. [PMID: 18090394]
German(a) P., Liu H. C., Szwarcberg J., et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr 2012;61(1):32-40. [PMID: 22732469]
German(b) P., Wei X., Mizuno V., et al. Pharmacokinetics of elvitegravir and cobicistat in subjects with severe renal impairment. 13 International Workshop on Clinical Pharmacology of HIV Therapy; 2012 Apr 16-18. https://www.natap.org/2012/pharm/Pharm_29.htm
Gervasoni C., Meraviglia P., Landonio S., et al. Low body weight in females is a risk factor for increased tenofovir exposure and drug-related adverse events. PLoS One 2013;8(12):e80242. [PMID: 24312465]
Goicoechea M., Liu S., Best B., et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis 2008;197(1):102-8. [PMID: 18171292]
Griffith D. C., Farmer C., Gebo K. A., et al. Uptake and virological outcomes of single- versus multi-tablet antiretroviral regimens among treatment-naïve youth in the HIV Research Network. HIV Med 2019;20(2):169-74. [PMID: 30561888]
Gulick R. M., Mellors J. W., Havlir D., et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 1997;337(11):734-39. [PMID: 9287228]
Gulick R. M., Mellors J. W., Havlir D., et al. 3-year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann Intern Med 2000;133(1):35-39. [PMID: 10877738]
Hanna D. B., Hessol N. A., Golub E. T., et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr 2014;65(5):587-96. [PMID: 24326606]
Hill A., Hughes S. L., Gotham D., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety?. J Virus Erad 2018;4(2):72-79. [PMID: 29682298]
Kakuda T. N., Opsomer M., Timmers M., et al. Pharmacokinetics of darunavir in fixed-dose combination with cobicistat compared with coadministration of darunavir and ritonavir as single agents in healthy volunteers. J Clin Pharmacol 2014;54(8):949-57. [PMID: 24644095]
Karras A., Lafaurie M., Furco A., et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis 2003;36(8):1070-73. [PMID: 12684922]
Koteff J., Borland J., Chen S., et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol 2013;75(4):990-96. [PMID: 22905856]
Kumar S., Rao P. S., Earla R., et al. Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol 2015;11(3):343-55. [PMID: 25539046]
Kuritzkes D. R., Lalama C. M., Ribaudo H. J., et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis 2008;197(6):867-70. [PMID: 18269317]
Lake J. E., Wu K., Bares S. H., et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis 2020;71(9):e471-77. [PMID: 32099991]
Lee F. J., Amin J., Carr A. Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks' follow-up. PLoS One 2014;9(5):e97482. [PMID: 24830290]
Lennox J. L., Landovitz R. J., Ribaudo H. J., et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014;161(7):461-71. [PMID: 25285539]
Lepist E. I., Zhang X., Hao J., et al. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int 2014;86(2):350-57. [PMID: 24646860]
Llibre J. M., Hill A. Abacavir and cardiovascular disease: a critical look at the data. Antiviral Res 2016;132:116-21. [PMID: 27260856]
Łomiak M., Stępnicki J., Mikuła T., et al. Weight and body mass index increase after switch from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate-containing treatment in an antiretroviral therapy-experienced group. Int J STD AIDS 2021;32(6):570-77. [PMID: 33612018]
Lundgren J. D., Babiker A. G., Gordin F., et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373(9):795-807. [PMID: 26192873]
Maggiolo F., Colombo G. L., Di Matteo S., et al. Cost-effectiveness analysis of antiretroviral therapy in a cohort of HIV-infected patients starting first-line highly active antiretroviral therapy during 6 years of observation. Patient Relat Outcome Meas 2015;6:53-60. [PMID: 25733942]
Maggiolo F., Di Filippo E., Valenti D., et al. NRTI sparing therapy in virologically controlled HIV-1 infected subjects: results of a controlled, randomized trial (Probe). J Acquir Immune Defic Syndr 2016;72(1):46-51. [PMID: 26910503]
Mallal S., Phillips E., Carosi G., et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008;358(6):568-79. [PMID: 18256392]
Marcus J. L., Neugebauer R. S., Leyden W. A., et al. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr 2016;71(4):413-19. [PMID: 26536316]
Marzolini C., Back D., Weber R., et al. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother 2011;66(9):2107-11. [PMID: 21680580]
McClung R. P., Oster A. M., Ocfemia M. C. B., et al. Transmitted drug resistance among human immunodeficiency virus (HIV)-1 diagnoses in the United States, 2014-2018. Clin Infect Dis 2022;74(6):1055-62. [PMID: 34175948]
McComsey G. A., Kitch D., Daar E. S., et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011;203(12):1791-1801. [PMID: 21606537]
Michienzi S. M., Schriever C. A., Badowski M. E. Abacavir/lamivudine/dolutegravir single tablet regimen in patients with human immunodeficiency virus and end-stage renal disease on hemodialysis. Int J STD AIDS 2019;30(2):181-87. [PMID: 30381029]
Mills A., Cohen C., Dejesus E., et al. Efficacy and safety 48 weeks after switching from efavirenz to rilpivirine using emtricitabine/tenofovir disoproxil fumarate-based single-tablet regimens. HIV Clin Trials 2013;14(5):216-23. [PMID: 24144898]
Mills(a) A., Arribas J. R., Andrade-Villanueva J., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 2016;16(1):43-52. [PMID: 26538525]
Mills(b) A., Fusco J., Schulman K., et al. The impact of antiretroviral tablet burden and polypharmacy on viral suppression in treatment naïve patients. Open Forum Infect Dis 2016;3(Suppl 1):1512. http://dx.doi.org/10.1093/ofid/ofw172.1214
Mocroft A., Lundgren J. D., Ross M., et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV 2016;3(1):e23-32. [PMID: 26762990]
Molina J. M., Andrade-Villanueva J., Echevarria J., et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet 2008;372(9639):646-55. [PMID: 18722869]
Molina J. M., Clotet B., van Lunzen J., et al. Once-daily dolutegravir is superior to once-daily darunavir/ritonavir in treatment-naive HIV-1-positive individuals: 96 week results from FLAMINGO. J Int AIDS Soc 2014;17(4 Suppl 3):19490. [PMID: 25393999]
Molina J. M., Squires K., Sax P. E., et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV 2018;5(5):e211-20. [PMID: 29592840]
Nachega J. B., Parienti J. J., Uthman O. A., et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014;58(9):1297-1307. [PMID: 24457345]
Obel N., Farkas D. K., Kronborg G., et al. Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: a population-based nationwide cohort study. HIV Med 2010;11(2):130-36. [PMID: 19682101]
Orkin C., DeJesus E., Khanlou H., et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med 2013;14(1):49-59. [PMID: 23088336]
Orkin C., Squires K. E., Molina J. M., et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis 2019;68(4):535-44. [PMID: 30184165]
Panichsillapakit T., Smith D. M., Wertheim J. O., et al. Prevalence of transmitted HIV drug resistance among recently infected persons in San Diego, CA 1996-2013. J Acquir Immune Defic Syndr 2016;71(2):228-36. [PMID: 26413846]
Perrot S., Aslangul E., Szwebel T., et al. Bone pain due to fractures revealing osteomalacia related to tenofovir-induced proximal renal tubular dysfunction in a human immunodeficiency virus-infected patient. J Clin Rheumatol 2009;15(2):72-74. [PMID: 19265350]
Pilkington V., Hughes S. L., Pepperrell T., et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate: an updated meta-analysis of 14 894 patients across 14 trials. AIDS 2020;34(15):2259-68. [PMID: 33048869]
Post F. A., Moyle G. J., Stellbrink H. J., et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010;55(1):49-57. [PMID: 20431394]
Pozniak A., Arribas J. R., Gathe J., et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr 2016;71(5):530-37. [PMID: 26627107]
Quesada P. R., Esteban L. L., Garcia J. R., et al. Incidence and risk factors for tenofovir-associated renal toxicity in HIV-infected patients. Int J Clin Pharm 2015;37(5):865-72. [PMID: 26008219]
Raboud J., Li M., Walmsley S., et al. Once daily dosing improves adherence to antiretroviral therapy. AIDS Behav 2011;15(7):1397-1409. [PMID: 20878227]
Raffi F., Babiker A. G., Richert L., et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet 2014;384(9958):1942-51. [PMID: 25103176]
Raffi F., Jaeger H., Quiros-Roldan E., et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013;13(11):927-35. [PMID: 24074642]
Rhee S. Y., Blanco J. L., Jordan M. R., et al. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med 2015;12(4):e1001810. [PMID: 25849352]
Ribaudo H. J., Benson C. A., Zheng Y., et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis 2011;52(7):929-40. [PMID: 21427402]
Ryom L., Mocroft A., Kirk O., et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis 2013;207(9):1359-69. [PMID: 23382571]
Saag M., Balu R., Phillips E., et al. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis 2008;46(7):1111-18. [PMID: 18444831]
Sabin C. A., Worm S. W., Weber R., et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008;371(9622):1417-26. [PMID: 18387667]
Sax P. E., Erlandson K. M., Lake J. E., et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020;71(6):1379-89. [PMID: 31606734]
Sax P. E., Pozniak A., Montes M. L., et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017;390(10107):2073-82. [PMID: 28867499]
Sax P. E., Tierney C., Collier A. C., et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis 2011;204(8):1191-1201. [PMID: 21917892]
Sax P. E., Tierney C., Collier A. C., et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med 2009;361(23):2230-40. [PMID: 19952143]
Sax P. E., Wohl D., Yin M. T., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015;385(9987):2606-15. [PMID: 25890673]
SMART/INSIGHT, D:A:D Study Groups . Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS 2008;22(14):f17-24. [PMID: 18753925]
Smith K. Y., Garcia F., Kumar P., et al. Assessing darunavir/ritonavir-based therapy in a racially diverse population: 48-week outcomes from GRACE. J Natl Med Assoc 2012;104(7-8):366-76. [PMID: 23092052]
Smith K. Y., Patel P., Fine D., et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS 2009;23(12):1547-56. [PMID: 19542866]
Souza S. J., Luzia L. A., Santos S. S., et al. Lipid profile of HIV-infected patients in relation to antiretroviral therapy: a review. Rev Assoc Med Bras (1992) 2013;59(2):186-98. [PMID: 23582562]
Stekler J. D., McKernan J., Milne R., et al. Lack of resistance to integrase inhibitors among antiretroviral-naive subjects with primary HIV-1 infection, 2007-2013. Antivir Ther 2015;20(1):77-80. [PMID: 24831260]
Stellbrink H. J., Orkin C., Arribas J. R., et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010;51(8):963-72. [PMID: 20828304]
Surial B., Mugglin C., Calmy A., et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med 2021;174(6):758-67. [PMID: 33721521]
Sweet D., Song J., Zhong Y., et al. Real-world medication persistence with single versus multiple tablet regimens for HIV-1 treatment. J Int AIDS Soc 2014;17(4 Suppl 3):19537. [PMID: 25394046]
Taiwo B., Zheng L., Gallien S., et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS 2011;25(17):2113-22. [PMID: 21857490]
Tashima K., Crofoot G., Tomaka F. L., et al. Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a phase IIIb, open-label single-arm trial. AIDS Res Ther 2014;11:39. [PMID: 25926858]
Trottier B., Machouf N., Thomas R., et al. Abacavir/lamivudine fixed-dose combination with ritonavir-boosted darunavir: a safe and efficacious regimen for HIV therapy. HIV Clin Trials 2012;13(6):335-42. [PMID: 23195671]
van Lunzen J., Antinori A., Cohen C. J., et al. Rilpivirine vs. efavirenz-based single-tablet regimens in treatment-naive adults: week 96 efficacy and safety from a randomized phase 3b study. AIDS 2016;30(2):251-59. [PMID: 26684822]
Venter W. D. F., Moorhouse M., Sokhela S., et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019;381(9):803-15. [PMID: 31339677]
Venter W. D. F., Sokhela S., Simmons B., et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV 2020;7(10):e666-76. [PMID: 33010240]
Wainberg M. A., Mesplede T. Implications for the future of the HIV epidemic if drug resistance against dolutegravir cannot occur in first-line therapy. J Int AIDS Soc 2015;18(1):20824. [PMID: 26642452]
Walmsley S., Baumgarten A., Berenguer J., et al. Brief report: Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015;70(5):515-19. [PMID: 26262777]
Walmsley S. L., Antela A., Clumeck N., et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013;369(19):1807-18. [PMID: 24195548]
Wang Z., Collura R. V., Rosenthal M., et al. Integrase genotypic testing and drug resistance among new HIV diagnoses in New York. CROI; 2019 Mar 4-7. http://www.croiconference.org/sessions/integrase-genotypic-testing-and-drug-resistance-among-new-hiv-diagnoses-new-york
Young B., Vanig T., Dejesus E., et al. A pilot study of abacavir/lamivudine and raltegravir in antiretroviral-naive HIV-1-infected patients: 48-week results of the SHIELD trial. HIV Clin Trials 2010;11(5):260-69. [PMID: 21126956]
Zack(a) J., Chuck S., Chu H., et al. Bioequivalence of the rilpivirine/emtricitabine/tenofovir alafenamide single-tablet regimen. J Bioequiv Availab 2016;8:49-54. https://doi.org/10.4172/jbb.1000266
Zack(b) J., Chu H., Chuck S., et al. Bioequivalence of two co-formulations of emtricitabine/tenofovir alafenamide fixed-dose combinations with 200/10 mg and 200/25 mg. J Bioequiv Availab 2016;8:68-73. https://doi.org/10.4172/jbb.1000270
Zash R., Holmes L. B., Diseko M., et al. Update on neural tube defects with antiretroviral exposure in the Tsepamo Study, Botswana. AIDS; 2022 Jul 29-Aug 2. https://www.natap.org/2022/IAC/IAC_31.htm
Zhang H., Shao Y., Garner W., et al. The effect of hepatic or renal impairment on bictegravir pharmokinetics. 18th International Workshop on Clinical Pharmacology of Antiviral Therapy; 2017 Jun 14-17. https://www.natap.org/2017/Pharm/Pharm_31.htm
Zimmermann A. E., Pizzoferrato T., Bedford J., et al. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis 2006;42(2):283-90. [PMID: 16355343]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | April 27, 2017 |
| Date of current publication | August 11, 2022 |
| Highlights of changes, additions, and updates in the August 11, 2022 edition |
The recommendation regarding discussion of the small risk of teratogenicity with DTG in the first trimester and the need for birth control while using DTG was removed. DTG has been shown to be safe throughout pregnancy. |
| Intended users | Clinicians in New York State who are initiating ART in nonpregnant, ART-naive adults with HIV |
| Lead author |
Antonio E. Urbina, MD |
| Contributing author(s) |
Nicole Bradley, BCPS, BCIDP; Yuman Lee, PharmD, BCIDP, AAHIVP; John M. Conry, PharmD, AAHIVP, FNAP |
| Writing group |
Steven M. Fine, MD, PhD; Rona Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Charles J. Gonzalez, MD; Christopher J. Hoffmann, MD, MPH |
| Author and writing group conflict of interest disclosures |
Antonio E. Urbina, MD: Scientific advisor: Gilead, Janssen, Merck, ViiV |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines | |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on January 18, 2024

