Purpose of This Guideline
Date of current publication: June 16, 2022
Lead author: Samuel T. Merrick, MD
Writing group: Steven M. Fine, MD, PhD; Rona Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Asa Radix, MD, MPH, PhD; Jessica Rodrigues; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: June 1, 2016
Periodic laboratory tests are necessary to evaluate a patient’s response to antiretroviral therapy (ART). Monitoring HIV-1 RNA levels (viral load) to confirm appropriate response to treatment and durable viral suppression is the most accurate and meaningful measure of the effectiveness of ART Gale, et al. 2013. HIV suppression is essential to the health of the individual with HIV and to preventing HIV transmission through sex.
Regular immunologic monitoring in patients with consistently undetectable HIV viral loads and CD4 counts >200 cells/mm3 offers little utility in clinical practice today. Clinicians rarely use this information to guide decision-making for clinically stable, virologically suppressed patients.
The New York State Department of Health AIDS Institute has developed these evidence-based recommendations for ambulatory care of patients with HIV to accomplish the following:
- Guide clinicians in the use of HIV viral load testing at appropriate times and intervals to assess initial and ongoing ART responses.
- Clarify the appropriate use of immunologic (CD4 count) monitoring in the care of patients with HIV.
Note on “experienced” and “expert” HIV care providers: Throughout this guideline, when reference is made to “experienced HIV care provider” or “expert HIV care provider,” those terms are referring to the following 2017 NYSDOH AI definitions:
- Experienced HIV care provider: Practitioners who have been accorded HIV Experienced Provider status by the American Academy of HIV Medicine or have met the HIV Medicine Association’s definition of an experienced provider are eligible for designation as an HIV Experienced Provider in New York State. Nurse practitioners and licensed midwives who provide clinical care to individuals with HIV in collaboration with a physician may be considered HIV Experienced Providers as long as all other practice agreements are met (8 NYCRR 79-5:1; 10 NYCRR 85.36; 8 NYCRR 139-6900). Physician assistants who provide clinical care to individuals with HIV under the supervision of an HIV Specialist physician may also be considered HIV Experienced Providers (10 NYCRR 94.2)
- Expert HIV care provider: A provider with extensive experience in the management of complex patients with HIV.
Viral Load and CD4 Count Monitoring Intervals
| RECOMMENDATIONS |
|
Monitoring Intervals
|
Very few studies address the appropriate frequency of viral load monitoring. A retrospective study noted that the strongest predictor of virologic failure at 12 months was a missed or canceled appointment rather than the interval of follow-up Buscher, et al. 2013. However, this and other similar studies Romih, et al. 2010; Reekie, et al. 2008 have significant limitations, including their retrospective nature and short follow-up periods. Data indicate that the linked sexual transmission of HIV in serodiscordant couples in which the partner with HIV maintains sustained viral suppression is negligible Rodger, et al. 2016.
Based on this information, those with HIV may rely on ART as a strategy to prevent viral transmission to an uninfected partner. Studies do not indicate the appropriate interval for viral suppression monitoring for ongoing transmission prevention. Until more definitive data are available, the decision to lengthen monitoring intervals for HIV RNA levels should be individualized. Patients who are monitored at longer intervals should be carefully selected based on length of viral suppression, CD4 count, use of ART for transmission prevention, and adherence to medical care, including visit attendance and retention in care.
| KEY POINT |
|
Table 1, below, provides a guide for monitoring HIV RNA levels and CD4 counts.
| Abbreviation: ART, antiretroviral therapy.
Notes:
|
|||
| Table 1: Recommended Viral Load and CD4 Count Monitoring in Nonpregnant Patients With HIV [a] | |||
| Event | HIV RNA Viral Load | CD4 Count | Comments |
| Entry into care | Baseline viral load (A1) | Baseline CD4 count (A1) |
|
| Patients Taking ART | |||
| ART initiation or change to address virologic failure |
|
|
|
| ART change for simplification or due to adverse effects | Within 4 weeks after ART change, then as below (A3) | Monitor as below for documented virologic suppression | — |
| Documented viral suppression |
|
|
— |
| New HIV RNA ≥500 copies/mL after previous viral suppression | Repeat viral load test 2 weeks after first result (A2) | Obtain CD4 count if previous result is >6 months old (B3) |
|
| New HIV RNA level over the limit of detection of sensitive assays, 20 to 50 copies/mL, but <500 copies/mL after previous viral suppression | Repeat viral load test within 4 weeks to differentiate low-level transient viremia (“blip”) from virologic failure [c] (A2) | If repeat viral load is detectable, obtain CD4 count if previous result is >6 months old (B3) |
|
| Patients Not Taking ART | |||
| CD4 count ≤500 cells/mm3 (A2) | At least every 4 months | At least every 4 months | At every visit, recommend ART initiation [b] |
| CD4 count >500 cells/mm3 (A2) | At least every 6 months | At least every 6 months | At every visit, recommend ART initiation [b] |
Virologic Monitoring (HIV Viral Load)
Plasma HIV-1 RNA level (viral load): Plasma levels of viral RNA have been shown to correlate with clinical outcomes, including overall mortality, and measurement of HIV RNA levels provides the most precise means of establishing whether a response to antiretroviral therapy (ART) has occurred HIV Surrogate Marker Collaborative Group 2000; Thiebaut, et al. 2000; Murray, et al. 1999; Marschner, et al. 1998; Mellors, et al. 1997.
HIV RNA levels should be obtained from all patients at baseline Porter, et al. 2015; Behrens, et al. 2014; Molina, et al. 2013; Tarwater, et al. 2004; Gulick, et al. 2003; Wu, et al. 2003.
For patients beginning ART, or those changing therapy as a result of virologic failure, HIV RNA should be measured at 4 weeks after initiation of ART and should decrease by at least 1 log (10-fold) in the presence of effective therapy Haubrich, et al. 2011 (see Table 2, below). For patients who do not have background antiretroviral resistance, an undetectable viral load (<50 copies/mL) is usually achieved within 3 months. Patients with a baseline HIV viral load >100,000 copies/mL can be expected to achieve an undetectable viral load within 6 months of effective treatment.
Table 2: Interpretation of Viral Load
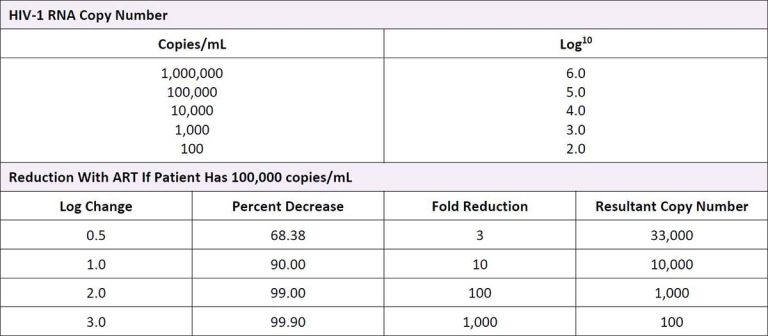
Abbreviation: ART, antiretroviral therapy.
Download Table 2: Interpretation of Viral Load Printable PDF
An absent or incomplete response of viral load to ART should raise concerns about poor adherence to therapy and/or viral resistance Townsend, et al. 2009; Baxter, et al. 2000.
Blips: Patients on previously suppressive ART with newly detectable HIV RNA levels of 50 to 500 copies/mL may be experiencing low-level transient viremia (“blip”) and not virologic failure. A blip by definition means that the viral load is again below the level of quantification on repeat testing performed promptly after a detectable result in someone previously suppressed. Persistent elevation, even at low levels, warrants further investigation. Acute concurrent illness and/or recent vaccination may cause this transient rise; however, studies have suggested that low-level transient viremia represents random biologic and statistical variation or false elevations of viral load resulting from laboratory processing Lee, et al. 2006; Nettles, et al. 2005. Blips are not known to be associated with the development of resistance mutations or virologic failure and do not require a change in ART Lee, et al. 2006. Retesting should be performed within 4 weeks to differentiate low-level transient viremia (a blip) from sustained viremia and possible virologic failure. The risk of virologic rebound (breakthrough) increases when values are >500 copies/mL Grennan, et al. 2012. However, ART should not be changed based on a single viral load elevation.
Advances in molecular detection technology have led to the development of HIV nucleic acid tests that are highly sensitive and more reliable than earlier versions. Real-time polymerase chain reaction (PCR) technology has been widely adopted for HIV-1 RNA quantification, but new technologies are continually emerging and being adapted to viral detection and quantification. The currently available HIV-1 viral load tests that use real-time PCR technology offer a larger dynamic range of quantification than early-version viral load tests. The lower and upper limits of quantification of the currently available U.S. Food and Drug Administration (FDA)-approved HIV-1 viral load tests are shown in Table 3, below. Several different HIV viral load tests have been developed, and 4 are currently approved for use in the United States.
| Abbreviations: FDA, U.S. Food and Drug Administration; LOQ, limit of quantification; NAT, nucleic acid test; PCR, polymerase chain reaction.
Note:
|
||
| Table 3: FDA-Approved Quantitative HIV-1 RNA Assays for Viral Load Monitoring | ||
| Test Name | Method | Lower and Upper LOQ |
| Abbott RealTime HIV-1 (Abbott Laboratories) | Real-time PCR |
|
| Cobas AmpliPrep/Cobas TaqMan HIV-1 Test, version 2.0 (Roche Diagnostics) | Real-time PCR |
|
| Cobas HIV-1 quantitative NAT for use on Cobas 6800/8800 systems (Roche Diagnostics) | Real-time PCR |
|
| Cobas TaqMan HIV-1 Test, v2.0 for use with the high pure system (Roche Diagnostics) | Real-time PCR |
|
All of the current FDA-approved viral load assays quantify the level of cell-free virus in an individual’s plasma and are approved for monitoring response to ART, tracking viral suppression, and detecting treatment failure. Successful ART should decrease the viral load by 1.5 to 2 logs (30- to 100-fold) within 6 weeks, with the viral load decreasing below the limit of detection within 6 months DHHS 2022. Cohort studies strongly suggest that patients with viral loads <50 copies/mL have more sustained viral suppression than patients with viral loads between 50 and 400 copies/mL. Assays that can detect <50 copies/mL are recommended for determining prolonged viral suppression and for monitoring patients who are on ART.
| KEY POINT |
|
Immunologic Monitoring (CD4 Count)
Lymphocyte subsets (CD4 count): CD4 lymphocyte count is used to evaluate immunologic staging, predict the risk of clinical progression, and make decisions regarding opportunistic infection prophylaxis Lopez Bernaldo de Quiros, et al. 2001; El-Sadr, et al. 2000. Low CD4 counts can be seen in other disease processes and should therefore not be used to diagnose HIV infection. Although CD4 count was used historically to establish a threshold for initiating ART, current guidelines in New York State recommend ART for all patients with HIV regardless of CD4 count. For patients who may not be ready to initiate ART, CD4 count can be used to guide discussions between patient and care provider regarding the urgency of initiating ART.
Although a CD4 count should be obtained at baseline Moore and Keruly 2007; Oldfield, et al. 1998; Havlir, et al. 1996; Schneider, et al. 1992; Fischl, et al. 1988, clinicians are unlikely to use it to guide clinical decision-making in practice for virologically suppressed patients once their CD4 count remains above 200 cells/mm3. However, for those infected with HIV-2 or HIV-1 variants that cannot be accurately quantified using viral load assays, CD4 count remains the most effective tool for monitoring disease progression.
Although a significant CD4 count increase often occurs among patients treated with effective ART, the absence of such an increase should not be interpreted as treatment failure if the viral load declines appropriately. ART regimens are generally not changed in patients with undetectable viral loads who experience immunologic failure, although patients should remain on appropriate prophylaxis for opportunistic infections based on CD4 count. One study of a cohort of more than 62,000 individuals in New York City over 1.9 years of observation reported that in those who entered the cohort with a CD4 count ≥350 cells/mm3, there was a >90% likelihood of sustaining a CD4 count >200 cells/mm3 during that period of time Myers, et al. 2016. Reassuringly, other data suggest that in patients with sustained viral suppression and CD4 counts between 100 and 200 cells/mm3, the risk of pneumocystis pneumonia is very low even in the absence of prophylaxis Chaiwarith, et al. 2013; Mocroft, et al. 2010; D'Egidio, et al. 2007.
Lack of correlation between viral load and CD4 count response is particularly common among patients ≥50 years old Sabin, et al. 2008; Gras, et al. 2007 and patients with low initial CD4 counts (<100 cells/mm3) Kelley, et al. 2009; Moore and Keruly 2007; Garcia, et al. 2004.
Absolute CD4 counts are calculated values that may fluctuate widely. The calculation is made by multiplying the total white blood cell count (in thousands) by the percentage of total lymphocytes and then by the percentage of CD4 lymphocytes. Therefore, any change in one of these three parameters will cause the absolute CD4 count to vary. CD4 percentage is a direct measurement and more reliable than the calculated absolute CD4 value, especially over time. A stable CD4 percentage, even when fluctuations occur in the absolute CD4 count, can reassure both the patient and the clinician that immunologic stability is present.
Some factors that can cause these fluctuations include sex, age, race, drugs (zidovudine, cephalosporins, cancer chemotherapy, nicotine, interferon, and corticosteroids), anti-lymphocyte antibodies, and splenectomy. Differences in reagents and equipment both within a laboratory and between laboratories may further contribute to variations in CD4 counts. There is also interlaboratory variation of normal range.
All Recommendations
| ALL RECOMMENDATIONS: VIROLOGIC AND IMMUNOLOGIC MONITORING IN HIV CARE |
|
Monitoring Intervals
|
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
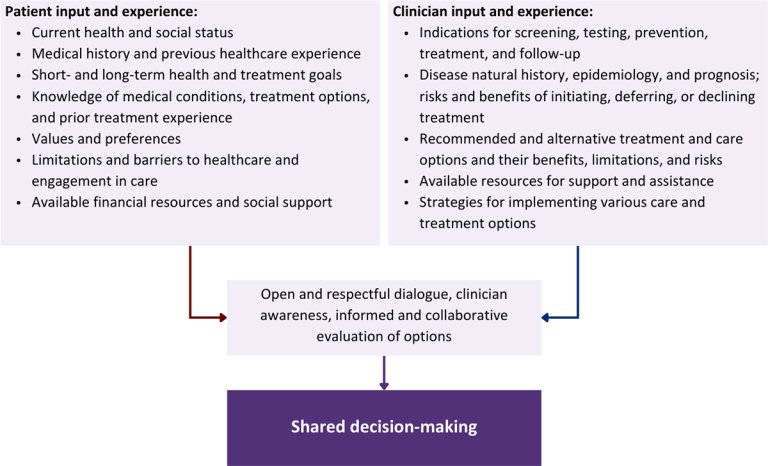
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Baxter J. D., Mayers D. L., Wentworth D. N., et al. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. CPCRA 046 Study Team for the Terry Beirn Community Programs for Clinical Research on AIDS. AIDS 2000;14(9):F83-93. [PMID: 10894268]
Behrens G., Rijnders B., Nelson M., et al. Rilpivirine versus efavirenz with emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected patients with HIV-1 RNA </=100,000 copies/mL: week 96 pooled ECHO/THRIVE subanalysis. AIDS Patient Care STDS 2014;28(4):168-75. [PMID: 24660840]
Buscher A., Mugavero M., Westfall A. O., et al. The association of clinical follow-up intervals in HIV-infected persons with viral suppression on subsequent viral suppression. AIDS Patient Care STDS 2013;27(8):459-66. [PMID: 23886048]
Chaiwarith R., Praparattanapan J., Nuntachit N., et al. Discontinuation of primary and secondary prophylaxis for opportunistic infections in HIV-infected patients who had CD4+ cell count <200 cells/mm(3) but undetectable plasma HIV-1 RNA: an open-label randomized controlled trial. AIDS Patient Care STDS 2013;27(2):71-76. [PMID: 23373662]
D'Egidio G. E., Kravcik S., Cooper C. L., et al. Pneumocystis jiroveci pneumonia prophylaxis is not required with a CD4+ T-cell count < 200 cells/microl when viral replication is suppressed. AIDS 2007;21(13):1711-15. [PMID: 17690568]
DHHS. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2022 Jan 20. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new-guidelines [accessed 2019 Sep 24]
El-Sadr W. M., Burman W. J., Grant L. B., et al. Discontinuation of prophylaxis against Mycobacterium avium complex disease in HIV-infected patients who have a response to antiretroviral therapy. Terry Beirn Community Programs for Clinical Research on AIDS. N Engl J Med 2000;342(15):1085-92. [PMID: 10766581]
Fischl M. A., Dickinson G. M., La Voie L. Safety and efficacy of sulfamethoxazole and trimethoprim chemoprophylaxis for Pneumocystis carinii pneumonia in AIDS. JAMA 1988;259(8):1185-89. [PMID: 3257532]
Gale H. B., Gitterman S. R., Hoffman H. J., et al. Is frequent CD4+ T-lymphocyte count monitoring necessary for persons with counts >=300 cells/muL and HIV-1 suppression?. Clin Infect Dis 2013;56(9):1340-43. [PMID: 23315315]
Garcia F., de Lazzari E., Plana M., et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr 2004;36(2):702-13. [PMID: 15167289]
Gras L., Kesselring A. M., Griffin J. T., et al. CD4 cell counts of 800 cells/mm. J Acquir Immune Defic Syndr 2007;45(2):183-92. [PMID: 17414934]
Grennan J. T., Loutfy M. R., Su D., et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 2012;205(8):1230-38. [PMID: 22438396]
Gulick R. M., Meibohm A., Havlir D., et al. Six-year follow-up of HIV-1-infected adults in a clinical trial of antiretroviral therapy with indinavir, zidovudine, and lamivudine. AIDS 2003;17(16):2345-49. [PMID: 14571186]
Haubrich R. H., Riddler S. A., Ribaudo H., et al. Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, nonnucleoside reverse transcriptase inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS 2011;25(18):2269-78. [PMID: 21941167]
Havlir D. V., Dube M. P., Sattler F. R., et al. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med 1996;335(6):392-98. [PMID: 8676932]
HIV Surrogate Marker Collaborative Group. Human immunodeficiency virus type 1 RNA level and CD4 count as prognostic markers and surrogate end points: a meta-analysis. HIV Surrogate Marker Collaborative Group. AIDS Res Hum Retroviruses 2000;16(12):1123-33. [PMID: 10954887]
Kelley C. F., Kitchen C. M., Hunt P. W., et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009;48(6):787-94. [PMID: 19193107]
Lee P. K., Kieffer T. L., Siliciano R. F., et al. HIV-1 viral load blips are of limited clinical significance. J Antimicrob Chemother 2006;57(5):803-5. [PMID: 16533823]
Lopez Bernaldo de Quiros J. C., Miro J. M., Pena J. M., et al. A randomized trial of the discontinuation of primary and secondary prophylaxis against Pneumocystis carinii pneumonia after highly active antiretroviral therapy in patients with HIV infection. Grupo de Estudio del SIDA 04/98. N Engl J Med 2001;344(3):159-67. [PMID: 11172138]
Marschner I. C., Collier A. C., Coombs R. W., et al. Use of changes in plasma levels of human immunodeficiency virus type 1 RNA to assess the clinical benefit of antiretroviral therapy. J Infect Dis 1998;177(1):40-47. [PMID: 9419168]
Mellors J. W., Munoz A., Giorgi J. V., et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997;126(12):946-54. [PMID: 9182471]
Mocroft A., Reiss P., Kirk O., et al. Is it safe to discontinue primary Pneumocystis jiroveci pneumonia prophylaxis in patients with virologically suppressed HIV infection and a CD4 cell count <200 cells/microL?. Clin Infect Dis 2010;51(5):611-19. [PMID: 20645862]
Molina J. M., Clumeck N., Redant K., et al. Rilpivirine vs. efavirenz in HIV-1 patients with baseline viral load 100,000 copies/ml or less: week 48 phase III analysis. AIDS 2013;27(6):889-97. [PMID: 23276806]
Moore R. D., Keruly J. C. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis 2007;44(3):441-46. [PMID: 17205456]
Murray J. S., Elashoff M. R., Iacono-Connors L. C., et al. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS 1999;13(7):797-804. [PMID: 10357378]
Myers J. E., Xia Q., Torian L. V., et al. Implementation and operational research: CD4 count monitoring frequency and risk of CD4 count dropping below 200 cells per cubic millimeter among stable HIV-infected patients in New York City, 2007-2013. J Acquir Immune Defic Syndr 2016;71(3):e73-78. [PMID: 26536317]
Nettles R. E., Kieffer T. L., Kwon P., et al. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA 2005;293(7):817-29. [PMID: 15713771]
Oldfield E. C., Fessel W. J., Dunne M. W., et al. Once weekly azithromycin therapy for prevention of Mycobacterium avium complex infection in patients with AIDS: a randomized, double-blind, placebo-controlled multicenter trial. Clin Infect Dis 1998;26(3):611-19. [PMID: 9524832]
Porter D. P., Kulkarni R., Fralich T., et al. 96-week resistance analyses of the STaR study: rilpivirine/emtricitabine/tenofovir DF versus efavirenz/emtricitabine/tenofovir DF in antiretroviral-naive, HIV-1-infected subjects. HIV Clin Trials 2015;16(1):30-38. [PMID: 25777187]
Reekie J., Mocroft A., Sambatakou H., et al. Does less frequent routine monitoring of patients on a stable, fully suppressed cART regimen lead to an increased risk of treatment failure?. AIDS 2008;22(17):2381-90. [PMID: 18981778]
Rodger A. J., Cambiano V., Bruun T., et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316(2):171-81. [PMID: 27404185]
Romih V., Zidovec Lepej S., Gedike K., et al. Frequency of HIV-1 viral load monitoring of patients initially successfully treated with combination antiretroviral therapy. PLoS One 2010;5(11):e15051. [PMID: 21124844]
Sabin C. A., Smith C. J., d'Arminio Monforte A., et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008;22(12):1463-73. [PMID: 18614870]
Schneider M. M., Hoepelman A. I., Eeftinck Schattenkerk J. K., et al. A controlled trial of aerosolized pentamidine or trimethoprim-sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus infection. The Dutch AIDS Treatment Group. N Engl J Med 1992;327(26):1836-41. [PMID: 1360145]
Tarwater P. M., Gallant J. E., Mellors J. W., et al. Prognostic value of plasma HIV RNA among highly active antiretroviral therapy users. AIDS 2004;18(18):2419-23. [PMID: 15622318]
Thiebaut R., Morlat P., Jacqmin-Gadda H., et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe d'Epidemiologie du SIDA en Aquitaine (GECSA). AIDS 2000;14(8):971-78. [PMID: 10853978]
Townsend D., Troya J., Maida I., et al. First HAART in HIV-infected patients with high viral load: value of HIV RNA levels at 12 weeks to predict virologic outcome. J Int Assoc Physicians AIDS Care (Chic) 2009;8(5):314-17. [PMID: 19759257]
Wu H., Mellors J., Ruan P., et al. Viral dynamics and their relations to baseline factors and longer term virologic responses in treatment-naive HIV-1-infected patients receiving abacavir in combination with HIV-1 protease inhibitors. J Acquir Immune Defic Syndr 2003;33(5):557-63. [PMID: 12902798]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | June 01, 2016 |
| Date of current publication | June 16, 2022 |
| Highlights of changes, additions, and updates in the June 16, 2022 edition |
Updated Table 1: Recommended Viral Load and CD4 Count Monitoring in Nonpregnant Patients With HIV |
| Intended users | Clinicians providing ambulatory care for patients with HIV |
| Lead author |
Samuel T. Merrick, MD |
| Writing group |
Steven M. Fine, MD, PhD; Rona Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Asa Radix, MD, MPH, PhD; Jessica Rodrigues, MS; Charles J. Gonzalez, MD; Christopher J. Hoffmann, MD, MPH |
| Author and writing group conflict of interest disclosures |
Joseph P. McGowan, MD, FACP, FIDSA: Institutional Pharma grant recipient/support, clinical trial; Gilead |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines |
Related NYSDOH AI Guidance |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on January 18, 2024

