Recommended Guidelines
Date of current publication: June 26, 2023
Lead author: Courtney Olson-Chen, MD
Writing group: Maria Teresa Timoney, MS, RN, CNM; Jessica Atrio, MD, MSc; Suzanne Kaufman, MPH, BSN, RN, AACRN
Date of original publication: August 10, 2020
Recommended guidelines: The Perinatal Transmission Prevention Guideline Committee of the New York State Department of Health AIDS Institute (NYSDOH AI) Clinical Guidelines Program recommends that clinicians who provide medical care for pregnant patients with HIV follow the Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States published by the U.S. Department of Health and Human Services (DHHS).
In addition to supporting the comprehensive DHHS recommendations, this committee also encourages clinicians in New York State to follow the good practices outlined below.
Note on “experienced” and “expert” HIV care providers: Throughout this guidance, when reference is made to “experienced HIV care provider” or “expert HIV care provider,” those terms are referring to the following 2017 NYSDOH AI definitions:
- Experienced HIV care provider: Practitioners who have been accorded HIV Experienced Provider status by the American Academy of HIV Medicine or have met the HIV Medicine Association’s definition of an experienced provider are eligible for designation as an HIV Experienced Provider in New York State. Nurse practitioners and licensed midwives who provide clinical care to individuals with HIV in collaboration with a physician may be considered HIV Experienced Providers as long as all other practice agreements are met (8 NYCRR 79-5:1; 10 NYCRR 85.36; 8 NYCRR 139-6900). Physician assistants who provide clinical care to individuals with HIV under the supervision of an HIV Specialist physician may also be considered HIV Experienced Providers (10 NYCRR 94.2)
- Expert HIV care provider: A provider with extensive experience in the management of complex patients with HIV.
| KEY POINT |
|
| LINKS TO CLINICAL RECOMMENDATIONS |
|
Key topics covered in the DHHS guideline Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States include the following: |
Antiretroviral Therapy
Do not interrupt antiretroviral therapy: Consistent adherence to antiretroviral therapy (ART) is essential to the maintenance of an undetectable HIV viral load in pregnant patients with HIV and is the best way to prevent perinatal transmission of HIV. Toward that end, this committee stresses that ART not be stopped or paused at any time antepartum, intrapartum, or postpartum, including any time when a patient has been directed to take nothing by mouth.
Any interruption in ART may increase the risk of perinatal transmission of HIV. Transmission rates as high as 18% have been reported when ART is interrupted in the third trimester Galli, et al. 2009. Continuing the baseline ART regimen intrapartum maximizes virologic suppression and reduces the risk of developing resistance to medications.
For clinical recommendations, see the DHHS guideline sections:
- Prepregnancy Counseling and Care for Persons of Childbearing Age With HIV
- Antepartum Care for Individuals With HIV
- Intrapartum Care for People With HIV
Continue ART postpartum: Good practice in discharge planning post-delivery includes making sure that the new parent has antiretroviral medications at home and has been counseled to avoid interruptions. HIV-associated clinical complications are reduced with the use of postpartum ART Currier, et al. 2017. For recommendations, see the DHHS guideline section Postpartum Follow-Up of People With HIV.
Syphilis Testing in the Third Trimester
Requirements: New York State Public Health Law requires that clinicians obtain serologic screening for syphilis for pregnant patients at the first prenatal visit and at delivery (cord blood testing). Syphilis screening between 28 and 32 weeks gestation is mandated by New York City. Effective May 3, 2024, New York State will require third trimester syphilis screening for all pregnant patients.
Recommendations: The NYSDOH AI recommends universal opt-out syphilis screening during the third trimester, preferably between 28 and 32 weeks gestation.
The incidence of primary and secondary syphilis among women has increased by 243% in the past 5 years in New York State NYSDOH 2023. Consequently, the number of cases of congenital syphilis is rising, with an increase from 29 cases in 2020 to 41 cases in 2021 NYSDOH 2023. Early third-trimester syphilis testing at 28 weeks is an additional opportunity for identifying syphilis and initiating treatment to reduce the risk of congenital syphilis. When diagnosed, syphilis is treated with penicillin G in collaboration with the local health department (see CDC: Syphilis Treatment Guideline, including Syphilis During Pregnancy).
HIV Testing for Sex Partners
Encourage HIV testing for sex partner(s): This committee encourages clinicians to recommend HIV testing for sex partners of pregnant patients. During the first prenatal visit, when a clinician provides counseling about HIV and other health conditions, the clinician can suggest that a patient’s sex partner(s) undergo testing for HIV. The same suggestion can be made if a patient reports having new sex partners during pregnancy. Any sex partner who injects drugs is at particularly high risk of acquiring HIV ACOG(b) 2018. Knowledge of sex partner HIV risk factors may be limited, as these individuals are not patients of the clinician. HIV testing of all sex partners would provide the most comprehensive evaluation to limit perinatal transmission. If a pregnant patient’s sex partner tests positive for HIV, initiation of ART will benefit the individual with HIV and will reduce the risk of sexual transmission. Barrier protection and pre-exposure prophylaxis (PrEP) may also be recommended for partners who test negative for HIV. Growing evidence suggests that involving partners in HIV treatment and prevention efforts during pregnancy can help decrease the risk of perinatal transmission Takah, et al. 2017.
Method of Delivery
Vaginal delivery with HIV viral load <1,000 copies/mL: Clinicians are strongly encouraged to perform HIV RNA testing within 4 weeks of a patient’s expected delivery date. If an HIV viral load <1000 copies/mL is detected, then a vaginal delivery as detailed in the DHHS guideline section Intrapartum Care for People With HIV is recommended, and intrapartum intravenous zidovudine may be considered if HIV viral load is between 50 and 999 copies/mL. Invasive fetal monitoring is best avoided in such individuals, with cesarean delivery reserved for the standard obstetrical indications.
Scheduled cesarean with HIV viral load ≥1,000 copies/mL: If a pregnant patient’s HIV viral load is ≥1000 copies/mL in the 4 weeks before delivery, a scheduled cesarean delivery is recommended ACOG(a) 2018. If there is no documented HIV RNA level within 4 weeks of delivery, cesarean delivery may be preferred in some cases, especially if the patient reports a lack of adherence to ART during pregnancy or has missed prenatal visits. Cesarean delivery is considered appropriate practice in such cases because this mode of delivery has been associated with a decreased risk of perinatal HIV transmission Andiman, et al. 1999. Without documented evidence of HIV viral suppression close to the time of delivery, there is a lack of reassurance that vaginal delivery would be a safe alternative to cesarean delivery. In pregnant patients who have a history of nonadherence with medical care, and particularly with ART, the potential for perinatal HIV transmission cannot be ignored, and a planned cesarean delivery before the onset of labor may be strongly considered. Clinical recommendations are provided in the DHHS guideline section Intrapartum Care for People With HIV.
Infant Feeding
A culturally sensitive, patient-centered approach to counseling about infant feeding that is evidence based and addresses patient and infant health and a patient’s values and desires is best to facilitate shared decision-making. When counseling a patient with HIV about infant feeding, discussion of alternatives to breastfeeding/chestfeeding, i.e., use of formula or pasteurized banked milk, that eliminate the risk of postnatal HIV transmission to the infant is advised. These replacement feeding methods are appropriate for patients with HIV who are not on ART or do not have a suppressed viral load, and clinicians can counsel such patients about the decreased risk of transmission (less than 1%) through breastfeeding/chestfeeding when viral suppression is achieved and maintained through consistent use of ART.
When a patient with HIV is on ART and has a sustained undetectable viral load and chooses to breastfeed/chestfeed, it is important for the care team (pediatric, adult, and obstetric) to support the patient’s decision. In such cases, the clinician can emphasize that adherence to the prescribed ART regimen reduces the risk of HIV transmission to the infant and that exclusive breastfeeding/chestfeeding up to age 6 months is preferable to feeding with a combination of breast milk and formula (mixed feeding). In addition to other small case reports from resource-rich settings, an evaluation of 10 infants who were exclusively breastfed/chestfed by mothers with HIV in the United States, there were no cases of infant HIV transmission Yusuf, et al. 2022.
Infant PrEP practices vary in the context of breastfeeding/chestfeeding. It is good practice for clinicians to consult an expert in pediatric HIV regarding the use of PrEP in infants being breastfed by a parent with HIV. Proposed harm reduction techniques beyond PrEP include exclusive breastfeeding/chestfeeding (as opposed to concurrent use of formula) and flash-heat treatment of expressed breast milk Levison, et al. 2014.
See the DHHS guideline section Infant Feeding for Individuals With HIV in the United States for complete evidence-based recommendations.
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
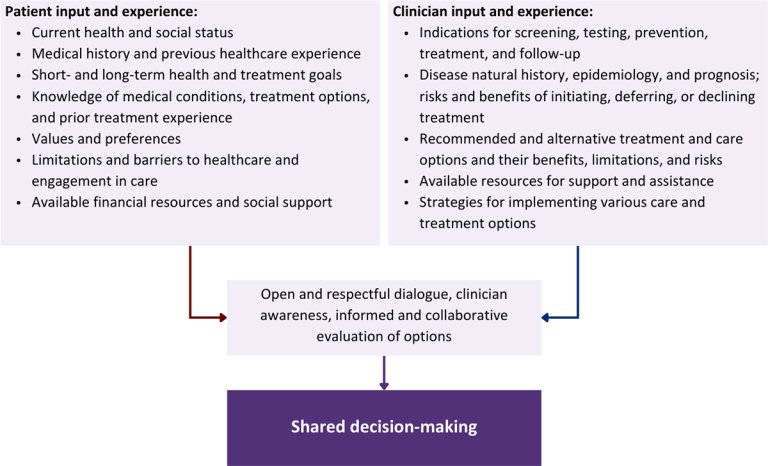
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
ACOG(a). ACOG committee opinion no. 751: labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol 2018;132(3):e131-37. [PMID: 30134427]
ACOG(b). ACOG committee opinion no. 752: prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol 2018;132(3):e138-42. [PMID: 30134428]
Andiman W., Bryson Y., de Martino M., et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1--a meta-analysis of 15 prospective cohort studies. N Engl J Med 1999;340(13):977-87. [PMID: 10099139]
Currier J. S., Britto P., Hoffman R. M., et al. Randomized trial of stopping or continuing ART among postpartum women with pre-ART CD4. PLoS One 2017;12(5):e0176009. [PMID: 28489856]
Galli L., Puliti D., Chiappini E., et al. Is the interruption of antiretroviral treatment during pregnancy an additional major risk factor for mother-to-child transmission of HIV type 1?. Clin Infect Dis 2009;48(9):1310-17. [PMID: 19309307]
Levison J., Weber S., Cohan D. Breastfeeding and HIV-infected women in the United States: harm reduction counseling strategies. Clin Infect Dis 2014;59(2):304-9. [PMID: 24771330]
NYSDOH. Sexually transmitted infections surveillance report, New York State, 2021. 2023 May 12. https://www.health.ny.gov/statistics/diseases/communicable/std/docs/sti_surveillance_report_2021.pdf [accessed 2023 May 19]
Takah N. F., Kennedy I. T. R., Johnman C. The impact of approaches in improving male partner involvement in the prevention of mother-to-child transmission of HIV on the uptake of maternal antiretroviral therapy among HIV-seropositive pregnant women in sub-Saharan Africa: a systematic review and meta-analysis. BMJ Open 2017;7(11):e018207. [PMID: 29175889]
Yusuf H. E., Knott-Grasso M. A., Anderson J., et al. Experience and outcomes of breastfed infants of women living with HIV in the United States: findings from a single-center breastfeeding support initiative. J Pediatric Infect Dis Soc 2022;11(1):24-27. [PMID: 34888664]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | August 10, 2020 |
| Date of current publication | June 26, 2023 |
| Highlights of changes, additions, and updates in the June 26, 2023 edition |
— |
| Intended users | NYS clinicians |
| Lead author |
Courtney Olson-Chen, MD |
| Writing group |
Maria Teresa Timoney, MS, RN, CNM; Jessica Atrio, MD, MSc; Suzanne Kaufman, MPH, BSN, RN, AACRN |
| Author and writing group conflict of interest disclosures | There are no author or writing group conflict of interest disclosures |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines | |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on October 27, 2023

