Purpose of This Guideline
Date of current publication: August 30, 2022
Lead author: Alok Gupta, MBBS
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD, FACP, AAHIVS; Charles J. Gonzalez, MD; Christopher J. Hoffmann, MD, MPH
Committee: Medical Care Criteria Committee
Date of original publication: August 1, 2013
Purpose: This guideline on prevention and management of hepatitis B virus (HBV) infection in adults with HIV has been developed by the New York State Department of Health AIDS Institute (NYSDOH AI) to guide clinicians in New York State who provide medical care for adults (≥18 years old) with HIV who are at risk of acquiring HBV or have HBV coinfection.
The goals of this guideline are to:
- Raise awareness among clinicians about the prevalence and associated risks of chronic HBV in patients with HIV.
- Increase screening for and vaccination against HBV in adults with HIV.
- Provide up-to-date, evidence-based recommendations on diagnosis, assessment, treatment, and monitoring of chronic HBV infection in patients with HIV, with emphasis on the essential components of antiretroviral therapy to treat coinfection.
HBV transmission: There are an estimated 1.25 to 2.49 million people with chronic HBV in the United States Lim, et al. 2020 and thousands of deaths annually from HBV-related complications, including cirrhosis and hepatocellular carcinoma (HCC) CDC(b) 2020. The primary routes of HBV transmission are perinatal transmission to the child, blood exposure, and sexual exposure. HBV DNA has been detected in various bodily secretions, including tears, urine, and saliva, but there is no firm evidence of HBV transmission via body fluids other than blood, semen, or vaginal secretions StatPearls 2022; Komatsu, et al. 2012.
Approximately 95% of individuals who acquire HBV in adulthood will mount an immune response, resulting in spontaneous recovery and production of protective HBV antibodies (anti‐HBs). However, some individuals will develop persistent HBV due to failure of the initial immune response to clear the virus, which results in chronic HBV infection Bennett, et al. 2019. Chronic HBV infection is defined as circulating hepatitis B surface antigen (HBsAg) in the blood for ≥6 months Terrault, et al. 2018.
HIV/HBV coinfection: HIV and HBV share similar transmission routes and both infections are often diagnosed in the same patients. Individuals with HIV born in the United States generally acquire HBV through sexual contact and injection drug use. In contrast, people with HIV born in HBV-endemic regions most commonly acquire the infection at birth or in early childhood Alter 2006.
In a large U.S. cohort study of individuals with HIV, from 1996 to 2007, 8.4% overall tested positive for HBsAg or detectable HBV DNA; prevalence was higher (10.3%) among men who have sex with men than among individuals who inject drugs (8.5%) and heterosexual individuals with risk factors (5.2%) Spradling, et al. 2010.
HIV/HBV coinfection can significantly influence the natural history, progression, management, morbidity, and mortality associated with both infections. HBV viremia and the risk of chronic HBV are increased in people with HIV Thio 2009. In addition, HIV infection is associated with decreased clearance of HBV e antigen. Individuals with HIV who acquire protective anti‐HBs through HBV infection remain at risk of developing low antibody levels and subsequent reactivation of HBV (reverse seroconversion). Individuals with HIV/HBV coinfection also tend to have a decreased inflammatory response to chronic HBV, indicated by decreased serum alanine transaminase levels, an increased risk of progression to cirrhosis and HCC, and increased mortality compared with individuals with HBV monoinfection Sun, et al. 2021; Pinato, et al. 2019; Singh, et al. 2017; Thio 2009.
Post-exposure prophylaxis: For recommendations on HBV post-exposure prophylaxis, see the NYSDOH AI guideline PEP to Prevent HIV Infection > Management of Potential Exposure to Hepatitis B Virus.
Note on “experienced” and “expert” HIV care providers: Throughout this guideline, when reference is made to “experienced HIV care provider” or “expert HIV care provider,” those terms are referring to the following 2017 NYSDOH AI definitions:
- Experienced HIV care provider: Practitioners who have been accorded HIV Experienced Provider status by the American Academy of HIV Medicine or have met the HIV Medicine Association’s definition of an experienced provider are eligible for designation as an HIV Experienced Provider in New York State. Nurse practitioners and licensed midwives who provide clinical care to individuals with HIV in collaboration with a physician may be considered HIV Experienced Providers as long as all other practice agreements are met (8 NYCRR 79-5:1; 10 NYCRR 85.36; 8 NYCRR 139-6900). Physician assistants who provide clinical care to individuals with HIV under the supervision of an HIV Specialist physician may also be considered HIV Experienced Providers (10 NYCRR 94.2)
- Expert HIV care provider: A provider with extensive experience in the management of complex patients with HIV.
HBV Screening and Diagnosis
| RECOMMENDATION |
Screening Tests
Diagnosis
Acute HBV Infection
Transmission Prevention
|
Abbreviations: ALT, alanine transaminase; anti-HBc, hepatitis B core antibody; anti-HBe, antibody to HBeAg; anti-HBs, hepatitis B surface antibody; ART, antiretroviral therapy; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IgG, immunoglobulin G; IgM, immunoglobulin M. |
| NEW YORK STATE LAW: REPORTING HBV INFECTION |
|
Screening Tests
Clinicians should screen all patients with HIV for HBV risk, vaccination history, and infection upon entry into medical care and perform baseline testing to determine HBV immune status. Initial laboratory testing includes serologic testing for HBsAg, anti-HBc total, and anti-HBs, with results interpreted as detailed in Table 1, below. Patients with anti-HBs levels of ≥10 IU/mL are considered immune to HBV DHHS 2022. If a patient with HIV decides against HBV vaccination and remains at risk, annual laboratory screening is recommended (see guideline section HBV Vaccination) Terrault, et al. 2018.
| Abbreviations: anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IgG, immunoglobulin G; IgM, immunoglobulin M. | ||||
| Table 1: Interpretation of HBV Screening Test Results | ||||
| HBsAg | Anti-HBs | Anti-HBc | Interpretation | |
| IgG | IgM | |||
| Negative | Negative | Negative | Negative | Susceptible to HBV infection |
| Negative | Positive | Negative | Negative | Immune due to HBV vaccination |
| Negative | Positive | Positive | Negative | Immune due to natural HBV infection |
| Positive | Negative | Positive | Positive | Acute HBV infection |
| Positive | Negative | Positive | Negative/ Positive |
Chronic HBV infection |
| Negative | Negative | Positive | Negative/ Positive |
Isolated anti-HBc positivity. Possible interpretations:
|
Download Table 1: Interpretation of HBV Screening Test Results Printable PDF
Diagnosis
For patients with positive HBsAg screening test results, follow-up laboratory testing should be performed to confirm HBV status (see Table 2, below). If a patient with HIV and unknown HBsAg status presents with signs or symptoms of acute hepatitis (i.e., elevated ALT), the clinician should perform HBsAg, anti-HBc IgM, HBeAg, anti-HBe, and HBV DNA testing to confirm a diagnosis.
| Abbreviations: anti-HBc, hepatitis B core antibody (IgG or IgM); anti-HBe, antibody to HBeAg; anti-HBs, hepatitis B surface antibody; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IgG, immunoglobulin G; IgM, immunoglobulin M.
Note:
|
|||||||
| Table 2: Serologic and Virologic Responses to HBV Infection | |||||||
| Stage of Infection | HBsAg | Anti-HBs | Anti-HBc IgG | Anti-HBc IgM | HBeAg | Anti-HBe | HBV DNA Level |
| Incubation | + | − | − | − | + or − | − | Low |
| Acute HBV infection | + | − | + | + | + | − | High |
| HBs-negative acute HBV | − | − | + | + | + or − | − | High |
| Inactive HBsAg carrier | + | − | +++ | + or − | − | + | Low |
| Precore mutant [a] | + | − | + or − | + or − | − | + | High |
| Occult infection | − | − | + | + or − | − | − | High or low |
| Chronic HBV infection | + | − | +++ | + or − | + or − | − | High or low |
Download Table 2: Serologic and Virologic Responses to HBV Infection Printable PDF
Acute HBV infection: Following exposure, HBV enters the bloodstream and circulates to the liver. The post-exposure time to the onset of abnormal liver enzymes averages 60 days (range, 40 to 90 days), and the onset of jaundice averages 90 days (range, 60 to 150 days). Acute HBV infection is asymptomatic in approximately 70% of patients, and <1% of patients develop fulminant hepatic failure. When symptoms manifest, they may include anorexia, malaise, nausea, vomiting, arthralgias, and right upper quadrant abdominal pain. Symptoms generally resolve within 4 weeks, with normalization of transaminase levels in 2 to 8 weeks.
Acute HBV infection is diagnosed through the detection of HBsAg and anti-HBc IgM. During the initial phase of infection, HBeAg and HBV DNA are also present (see Figure 1, below). Recovery is marked by the disappearance of HBV DNA and seroconversion of HBeAg to anti-HBe and of HBsAg to anti-HBs Shiffman 2010.
Figure 1: Typical Serologic Course of Acute Hepatitis B Virus Infection With Recovery
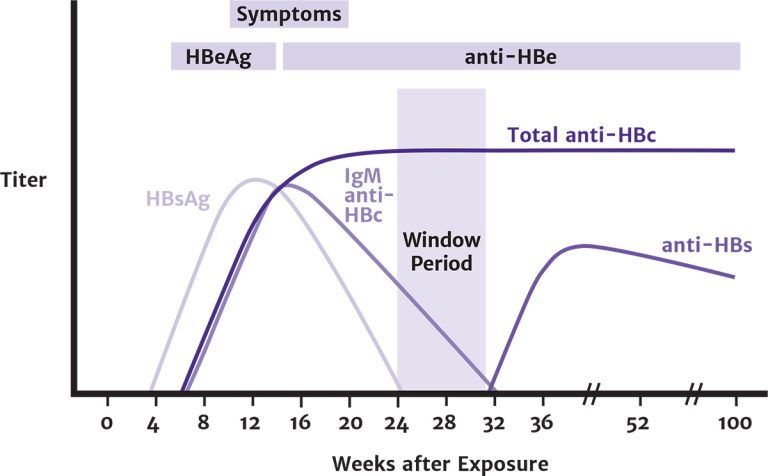
Abbreviations: anti-HBc, hepatitis B core antibody; anti-HBe, antibody to HBeAg; anti-HBs, hepatitis B surface antibody; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; IgM, immunoglobulin M.
Reprinted from Centers for Disease Control and Prevention Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection
Download figure: Typical Serologic Course of Acute Hepatitis B Virus Infection With Recovery
The ideal management strategy for symptomatic acute HBV infection in patients with HIV is not clear. Treatment with entecavir (ETV), tenofovir alafenamide (TAF), or tenofovir disoproxil fumarate (TDF) outside of a fully active anti-HIV regimen could lead to HIV resistance, but initiation of a fully active ART regimen may worsen acute liver disease due to immune reconstitution inflammatory syndrome (IRIS). For most patients with acute HBV, treatment is mainly supportive. Antiviral therapy is generally not indicated in patients with symptomatic acute HBV because most immunocompetent adults with acute HBV recover spontaneously.
However, antiviral treatment is indicated for patients with acute liver failure or a protracted, severe course of HBV, as indicated by a total bilirubin level >3 mg/dL (or direct bilirubin level >1.5 mg/dL), an international normalized ratio >1.5, encephalopathy, or ascites. ETV, TAF, and TDF are the preferred antiviral agents for these patients. Treatment should be continued until HBsAg clearance is confirmed or should be continued indefinitely in patients who undergo liver transplantation Terrault, et al. 2018. Patients with HIV who are already taking a fully active ART regimen that includes TAF or TDF should continue with the regimen.
If acute HBV infection is confirmed in an asymptomatic patient, the clinician should repeat ALT testing within 2 to 4 weeks to assess for symptoms of liver disease progression and repeat HBsAg, HBeAg, anti-HBe, and HBV DNA testing in 6 months to determine whether infection has cleared. Patients with symptomatic acute HBV require more frequent monitoring tailored to the patient’s condition.
Chronic HBV infection: HBV infection is a dynamic disease, and individuals can transition through the defined clinical phases with variable levels of serum ALT activity, HBV DNA, and HBV antigens. See the guideline sections Assessment Before HBV Treatment and HBV Treatment and Monitoring for recommendations on the management of chronic HBV infection in patients with HIV.
Reactivation: Chronic HBV can resolve in some patients, and tests will indicate a sustained loss of HBsAg, undetectable serum HBV DNA levels, and absence of clinical or histologic evidence of active viral infection. However, reactivation of HBV replication, characterized by the reappearance of HBeAg and HBsAg and a rise in serum HBV DNA, can occur. Reactivation is usually seen in patients taking immunosuppressive therapy for a concurrent medical condition; in rare instances, patients with prior resolved HBV infection who are anti-HBs-positive can have reactivation of HBV during subsequent immunosuppressive therapy. For a list of medications associated with increased risk for HBV reactivation, see Medscape > Hepatitis B Treatment & Management. Reactivation of HBV can also occur in individuals with HIV, including those who experience immune reconstitution after initiation of ART. HBV reactivation may result in severe hepatitis and should be considered a potential cause of hepatitis in patients with previously resolved HBV infection. During reactivation, serum ALT levels will be elevated, and patients who were HBeAg- or HBsAg-negative may become both HBeAg- and HBsAg-positive. HBV reactivation can vary from mild and asymptomatic to severe with possible fulminant hepatic failure.
Occult HBV infection is defined as detectable HBV DNA in HBsAg-negative patients. Most patients with occult HBV have very low or undetectable serum levels, but HBV DNA is often detected in the liver. Patients with occult infection are at risk of HBV reactivation if they receive potent immunosuppressive therapy or chemotherapy. Occult HBV infection has been associated with chronic liver disease and increased risk of hepatocellular carcinoma Raimondo, et al. 2007.
Transmission Prevention
HBV is significantly more transmissible through exposure to blood and body fluid than HIV and requires more frequent assessment for behaviors that increase HIV/HBV transmission risk. Barrier protection, including latex or polyurethane condoms, should be recommended to decrease the risk of sexual transmission Smith, et al. 2015; Weller and Davis 2002, and sexual partners should be vaccinated if possible. It is important to advise patients that household contacts should be vaccinated against HBV and that they should avoid sharing any objects that may be contaminated with blood, such as razors or toothbrushes.
All active injection drug users should be prescribed clean syringes and needles and offered referrals to substance use treatment, such as opioid substitution. Referral to needle-exchange programs should also be offered (see NYSDOH Drug Use Resources). Injection drug users should also receive information about safe disposal and storage of needles/syringes and safer injection techniques.
Figure 2: Algorithm for HBV Screening and Vaccination in Patients With HIV
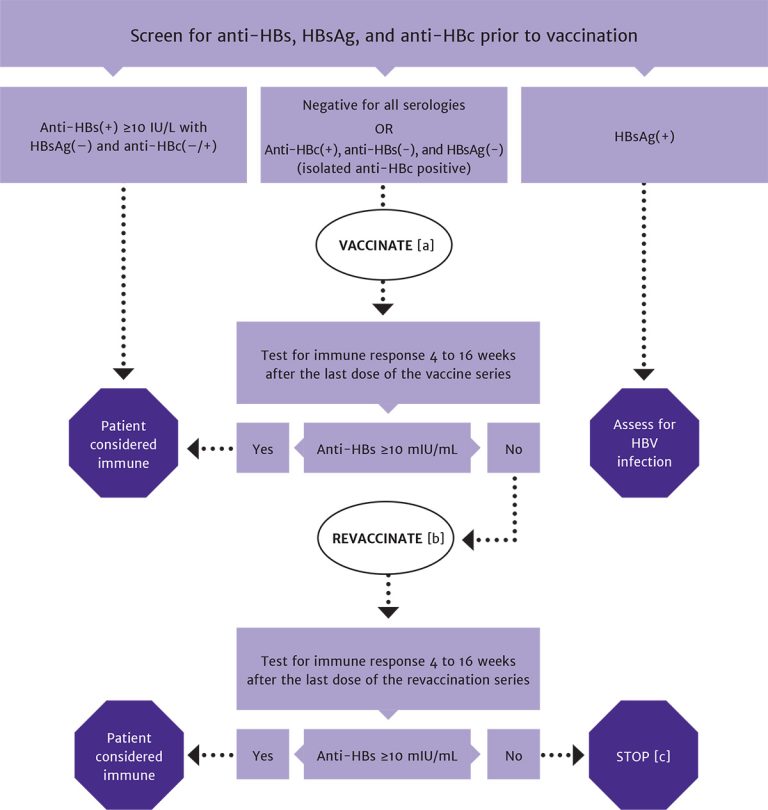
Abbreviations: anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
Notes:
- In patients with positive anti-HBc, negative anti-HBs, and negative HBsAg test results, vaccinate with 1 standard dose of HBV vaccine and check anti-HBs titer after 8 weeks. If titer is <100 mlU/mL, complete remaining doses in the vaccine series and recheck titer 8 weeks after the last vaccine.
- In patients with anti-HBs levels <10 mlU/mL (vaccine nonresponse), revaccination is recommended with the Heplisav-B vaccine series or a double dose of the vaccine series previously administered.
- A patient who is negative for all serologies and who does not respond to revaccination may have a primary nonresponse or chronic infection. HBV DNA testing may be used to detect the presence of chronic HBV infection.
Download figure: Algorithm for HBV Screening and Vaccination in Patients With HIV
HBV Vaccination
| RECOMMENDATIONS |
Primary Vaccination
Revaccination
|
Abbreviations: anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus. |
Vaccination effectively prevents HBV infection. Patients with HIV with negative anti-HBs, anti-HBc, and HBsAg test results have no evidence of immunity and should be offered vaccination against HBV DHHS 2022; see Figure 2: Algorithm for HBV Screening and Vaccination in Patients With HIV and Table 1: Interpretation of HBV Screening Test Results. Conversely, patients with positive anti-HBc and anti-HBs test results have resolved HBV infection and do not require vaccination.
Primary Vaccination Strategies
The single-antigen HBV vaccines currently approved by the U.S. Food and Drug Administration (FDA) for individuals ≥18 years old are Engerix-B, Recombivax HB, and Heplisav-B. Prehevbrio, a new 3-antigen recombinant HBV vaccine, was approved in 2021 by the FDA for use for individuals ≥18 years old FDA 2021, but experience regarding its use in patients with HIV is lacking at this time.
The level of immune response to HBV vaccination in individuals with HIV can be lower than in adults who are HIV seronegative Mast, et al. 2006; Rey, et al. 2000; Tayal and Sankar 1994; Loke, et al. 1990. Many studies have shown that the presence of detectable HIV RNA Overton, et al. 2005; Tedaldi, et al. 2004 and low CD4 cell counts Veiga, et al. 2006; Fonseca, et al. 2005; Tedaldi, et al. 2004; Keet, et al. 1992 correlates with a poor immune response to vaccination. Ideally, based on the data, the HBV vaccine should be administered before a patient’s CD4 count declines to <350 cells/mm3 to improve immunogenicity; however, vaccination should not be deferred in patients who have CD4 counts <350 cells/mm3.
The initial vaccine series using conventional HBV vaccines (Engerix-B, Recombivax HB) is typically administered intramuscularly as 3 standard doses at 0, 4, and 24 weeks (see Table 3, below). Whether patients with HIV should receive a standard or double dose of these vaccines is still being debated. This committee and the DHHS Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV recommend administering the standard 3-dose regimen. If the patient does not respond, a higher dose can be administered when the patient is revaccinated DHHS 2022.
Heplisav-B, a 2-dose (4 weeks apart) recombinant HBsAg vaccine with a novel adjuvant, is also available for individuals ≥18 years old FDA 2020. In 3 randomized controlled trials among individuals without HIV, administration of 2 doses of Heplisav-B was associated with a higher seroprotection rate than 3 doses of Engerix-B FDA 2020.
A recent retrospective cohort study among individuals with HIV found seroprotection rates were increased with the Heplisav-B vaccine compared with other previously used HBV vaccines Schnittman, et al. 2021. In addition, a recent modeling study determined that use of Heplisav-B among individuals with HIV results in lower costs and increased benefits compared with Engerix-B Rosenthal, et al. 2020. A 2-dose series may increase adherence because it requires fewer follow-up visits and a shorter wait time between doses than the 3-dose vaccines. No data are available to support use of other recombinant vaccines for the second dose if Heplisav-B is used for the initial dose.
Combined HBV and hepatitis A virus (HAV) vaccine: Twinrix is a combination vaccine that includes recombinant HBV and HAV vaccines; it is approved by the FDA for use in individuals ≥18 years old in the United States. For individuals with HIV who are not immune to both HAV and HBV, Twinrix may be administered as an initial series and administered in 3 doses at 0, 4, and 24 weeks. No data are available to support the administration of this vaccine as a double-dose or 4-dose series, so these strategies are not recommended in patients with HIV.
| Abbreviations: ESRD, end-stage renal disease; HAV, hepatitis A virus; HBV, hepatitis B virus; IM, intramuscular.
Notes:
|
||
| Table 3: HBV Vaccine Dosing Schedule | ||
| Vaccine | Dosing | Notes |
| Engerix-B | Single dose: 20 µg as one 1 mL dose containing 20 µg/mL vaccine administered as follows:
Double dose: 40 µg as two 1 mL doses of 20 µg/mL vaccine administered as follows:
|
Patients with ESRD or other immunocompromising conditions [a]: 40 µg/mL as two 1 mL doses of 20 µg/mL vaccine administered in 3 IM injections at weeks 0, 4, 8, and 24 |
| Recombivax HB | Single dose: 10 µg as one 1 mL dose containing 10 µg/mL vaccine administered as follows:
Double dose [b]: 20 µg as two 1 mL doses containing 10 µg/mL vaccine administered as follows:
|
Patients with ESRD or other immunocompromising conditions [a]: 40 µg/mL as 1 mL of higher-strength vaccine administered in 4 IM injections at weeks 0, 4, and 24 |
| Heplisav-B [c] | Single dose: 20 µg as one 0.5 mL dose containing 20 µg/mL vaccine administered as follows:
|
|
| Twinrix [d] | Single dose: 1 mL administered as follows:
|
For patients who are not immune to either HBV or HAV |
Double-dose and 4-dose strategies: Other vaccination approaches are to administer a double dose of vaccine on a standard 3-dose schedule or to add a fourth dose at 2 months to a 3-dose vaccine series. Several studies have shown improved immune response to double-dose vaccinations given in a 3-dose schedule Psevdos, et al. 2010; de Vries-Sluijs, et al. 2008; Fonseca, et al. 2005. A 2013 meta-analysis (5 studies, n=883) found that increasing the vaccine dosage may significantly improve immune responses in participants with HIV Ni, et al. 2013. A 2015 multicenter, open-label, randomized controlled trial (RCT) compared standard-dose (20 μg) with double-dose (40 μg) HBV revaccination in adults who did not respond to primary vaccination Rey, et al. 2015. In this study, double-dose revaccination was not associated with a higher response rate than revaccination with a standard single-dose regimen at week 4 after vaccination. However, the proportion who responded to the vaccine and the geometric mean titers at week 4 after vaccination were higher in the double-dose group than in the standard-dose group. Seroprotective responses at week 72 were greater in the double-dose group than in the standard-dose group. The safety profile was similar between the groups Rey, et al. 2015.
An RCT conducted in 2013 compared the immunogenicity and safety of 4 standard doses and 4 double doses with 3 standard doses of HBV vaccination in adults with HIV Chaiklang, et al. 2013. Response rates were higher in the 4-dose group than in the standard 3-dose group, but the difference was not statistically significant. Local adverse effects were more common with increased frequency and dosage of vaccine, but systemic and serious adverse effects were extremely rare Chaiklang, et al. 2013. Based on these data, it is reasonable to consider an alternative primary HBV vaccination approach with a 3- or 4-injection double-dose vaccine series in patients with HIV.
Accelerated vaccination: An RCT using the standard-dose HBV vaccine compared an accelerated schedule (0, 1, and 3 weeks) with the standard schedule (0, 4, and 24 weeks) and demonstrated a noninferior response rate for participants with CD4 counts >500 cells/mm3; this schedule may increase patient adherence to the full vaccine series de Vries-Sluijs, et al. 2011. However, the accelerated schedule was inferior in patients with CD4 counts of 200 to 500 cells/mm3. Because of the low number of participants with CD4 counts <200 cells/mm3, the results were inconclusive for this population.
Based on these findings, the accelerated schedule may be considered for patients with CD4 counts ≥500 cells/mm3 but is not recommended for patients with CD4 counts <500 cells/mm3 de Vries-Sluijs, et al. 2011. If an accelerated HBV vaccination schedule is used, the patient should also receive a fourth-dose booster at least 6 months after initiation of the vaccine series.
Pregnancy: Clinicians should not defer initial vaccination or revaccination in pregnant patients with HIV who do not have immunity to HBV. There are no well-controlled studies designed to evaluate the recommended anti-HBV vaccines during pregnancy. However, available data do not suggest an increased risk of miscarriage or major congenital disabilities in individuals who received Engerix-B, Twinrix, Recombivax HB, or Heplisav-B vaccines during pregnancy compared with individuals in the general U.S. population who were not vaccinated during pregnancy FDA 2020; FDA(a) 2018; FDA(b) 2018; FDA(c) 2018.
Isolated anti-HBc positivity: Defined as having negative HBsAg, negative anti-HBs, and positive anti-HBc test results, isolated anti-HBc positivity has been reported in 0.4% to 1.7% of blood donors in low prevalence areas and 10% to 20% of the population in endemic countries Lok, et al. 1988. It has been estimated that 17% to 41% of patients with HIV have isolated anti-HBc positivity Bhattacharya, et al. 2016; Witt, et al. 2013; Neau, et al. 2005. As shown in Table 1: Interpretation of HBV Screening Test Results, there are 4 possible interpretations of this result: resolved HBV infection with waning anti-HBs titers, false-positive result, occult HBV infection, or resolving acute HBV infection Mast, et al. 2006.
Most patients with HIV and isolated anti-HBc positivity are HBV DNA-negative, not immune to HBV Gandhi, et al. 2005, and routinely checking HBV DNA is no longer recommended. Clinicians should offer patients with HIV and isolated anti-HBc a single standard dose of HBV vaccine DHHS 2022. Anti-HBs testing should be performed 8 weeks after the first dose. If the anti-HBs titer is <100 mIU/mL, the remaining vaccines in the series should be administered, and anti-HBs testing should be repeated 8 weeks after the vaccine series is complete DHHS 2022; Piroth, et al. 2016. In a prospective study of 54 patients with HIV and isolated anti-HBc, 46% responded to a single dose of vaccine. Of those who did not respond to a single dose, 89% developed immunity after a 3-dose series of double-dose vaccine Piroth, et al. 2016. For patients with an anti-HBs titer ≥100 mIU/mL, clinicians may opt to discontinue the vaccine series. There are few data to guide the optimal number of vaccine doses for these patients but no evidence of harm in completing the full vaccination series.
However, if patients with HIV and isolated anti-HBc refuse vaccination or if post-vaccination anti-HBs testing cannot be assured, then a reasonable approach is to perform HBV DNA testing Chang, et al. 2018. HBV DNA testing may also be performed in patients with isolated anti-HBc who do not respond to the full vaccine series. A positive HBV DNA test result in a patient with isolated anti-HBc test results indicates occult HBV infection (see guideline section HBV Screening and Diagnosis > Diagnosis > Reactivation).
Follow-up Testing
Clinicians should repeat anti-HBs testing 4 to 16 weeks, based on the patient’s visit schedule, after vaccination to ensure immunity Rubin, et al. 2014. If the anti-HBs titer is ≥10 mIU/mL, the patient is considered immune to HBV. If the anti-HBs titer is <10 mIU/mL, the patient may have primary nonresponse to the vaccine and require revaccination, or the patient may have chronic HBV infection. HBV DNA testing may be used to detect chronic HBV.
| KEY POINT |
|
Revaccination
Individuals with HIV who do not respond (anti-HBs <10 mIU/mL) to the primary HBV vaccine series should be revaccinated with Heplisav-B or a double dose of the vaccine series previously administered. In a recent retrospective, cross-sectional study among individuals with HIV who failed to seroconvert after vaccination (HBsAg- and anti-HBs-negative) with Engerix-B or Recombivax HB, revaccination with Heplisav-B was highly effective in achieving seroprotection Khaimova, et al. 2021. If Heplisav-B is not administered as the initial HBV vaccination series, revaccination with the 2-dose series may be considered.
If the primary HBV vaccination was a 3-dose series, a 4-dose series may be considered. Compared with a single-dose vaccine series for revaccination, a double-dose HBV vaccine series for revaccination improved immune response in some individuals with HIV Rey, et al. 2015; Chaiklang, et al. 2013; Launay, et al. 2011; de Vries-Sluijs, et al. 2008.
Revaccination can be deferred for patients initiating ART until the CD4 count is ≥200 cells/mm3; response rates to vaccination may be higher in patients with CD4 counts ≥200 cells/mm3 than those with lower CD4 cell counts Gandhi, et al. 2005.
Assessment Before HBV Treatment
| RECOMMENDATIONS |
Liver Disease Assessment
Alcohol Use Screening and Education
HAV, HCV, and HDV Status
|
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CBC, complete blood count; DAA, direct-acting antiviral; HAV, hepatitis A virus; HBV, hepatitis B virus; HCC; hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus; IgG, immunoglobulin G; IgM, immunoglobulin M; PT/INR, prothrombin time/international normalized ratio. Note:
|
Liver Disease Assessment
Initial assessment of patients with chronic HBV should include a detailed history and physical examination to evaluate for any signs of advanced liver disease, including bruising, jaundice, dark urine, light stools, history of gastrointestinal bleeding, and pruritus. A prior treatment history, including medication history, should be obtained to determine whether the patient has previously taken hepatotoxic medications or lamivudine or emtricitabine, which have been associated with HBV resistance when taken as monotherapy. On examination, identify any stigmata of advanced liver disease, such as spider angiomas, splenomegaly, palmar erythema, and asterixis. The presence of ascites or encephalopathy indicates decompensated liver disease. Baseline laboratory tests include CBC, albumin, bilirubin, alkaline phosphatase, prothrombin time, ALT, and AST. Low albumin levels or elevated prothrombin time may suggest advanced liver disease with hepatic decompensation. Leukopenia and thrombocytopenia may indicate the presence of portal hypertension.
All individuals should be evaluated for liver fibrosis using noninvasive methods, such as transient elastography (FibroScan), serum testing for biomarkers (FibroSure), or AST to platelet ratio index (APRI) calculation. Liver biopsy is no longer preferred because of the risk of complications (e.g., bleeding, infection) and the possibility of a sampling error when only a small portion of the liver is evaluated. All patients with HIV/HBV coinfection should have a baseline ultrasound to screen for HCC Terrault, et al. 2018, and those with cirrhosis should be referred to a hepatologist to screen for esophageal varices Garcia-Tsao, et al. 2017; de Franchis 2015.
Results of the liver disease assessment determine the phase of chronic HBV infection. Liver biopsy results are included below as part of the description of each stage. However, liver biopsy is rarely indicated in patients with HIV/HBV. The procedure can be considered in patients who have persistently elevated ALT but persistently low HBV DNA to exclude other causes of liver disease.
- Immune tolerance: Characterized by hepatitis B e antigen (HBeAg) positivity with elevated HBV DNA levels but normal or minimally elevated ALT levels. Liver biopsies are generally benign, without signs of necroinflammation or fibrosis Tran 2011.
- Immune active: Subdivided into HBeAg-positive and HBeAg-negative. In HBeAg-positive patients, HBV DNA levels are typically >20,000 IU/mL, and serum ALT levels are elevated. In HBeAg-negative patients, HBV DNA levels tend to be lower (2,000 to 20,000 IU/mL) with low to normal serum ALT levels. Liver biopsy often reveals chronic hepatitis with variable signs of necroinflammation or fibrosis Terrault, et al. 2018.
- Inactive chronic HBV: These patients are HBeAg-negative and antibody to HBeAg-positive. Serum HBV DNA is usually <2000 IU/mL or undetectable, and ALT levels are normal. Liver biopsy indicates an absence of significant necroinflammation and variable levels of fibrosis Terrault, et al. 2018.
Alcohol Use Screening and Education
In 2020, there were an estimated 29,000 deaths from alcoholic liver disease and 51,000 deaths from chronic liver disease and cirrhosis in the general U.S. population CDC(a) 2022; CDC(b) 2022. Chronic alcohol use in patients with HBV infection results in increased oxidative stress and liver inflammation, which can progress to cirrhosis and lead to the development of HCC Donato, et al. 1997; Nakanuma and Ohta 1983. These effects are even more pronounced in patients with HIV/HBV coinfection in whom increased levels of liver inflammation, liver fibrosis, drug-induced hepatotoxicity, liver cirrhosis, and death from liver disease and HCC have been observed Marcellin, et al. 2008; Poynard, et al. 2003; Núñez, et al. 2001. Patient education is essential to helping patients understand the effects of alcohol use on the course of HBV infection, as is counseling for patients with underlying liver disease so that patients can make informed decisions regarding alcohol use or abstinence. Studies have shown that individual counseling and peer group education and support can be effective in reducing alcohol use in patients with HIV Knox, et al. 2013; Velasquez, et al. 2009.
HAV, HCV, and HDV Status
HAV: For information on HAV/HIV coinfection, see the NYSDOH AI guideline Prevention and Management of Hepatitis A Virus Infection in Adults With HIV > Management of HAV/HIV Coinfection.
HCV: HBV/HCV coinfection is associated with higher rates of cirrhosis, increased severity of liver disease, and increased risk of HCC than HBV or HCV monoinfection Mavilia and Wu 2018. This is of particular concern in patients with HIV/HBV coinfection; patients with HIV infection have more severe liver disease and higher rates of liver complications than patients without HIV Bräu, et al. 2007; Thio, et al. 2002. For information on screening, diagnosis, and treatment of HCV in patients with HIV, see the NYSDOH AI guidelines Hepatitis C Virus Screening, Testing, and Diagnosis in Adults and Treatment of Chronic Hepatitis C Virus Infection in Adults.
HDV: Formerly known as hepatitis delta virus, HDV is a defective satellite RNA virus that requires active HBV infection to replicate. HIV/HBV/HDV tri-infection is associated with more rapid liver disease progression and higher rates of decompensated cirrhosis, HCC, and mortality than HIV/HBV coinfection [Béguelin, et al. 2017; Fernández-Montero, et al. 2014; Castellares, et al. 2008; Sheng, et al. 2007]. HDV infection is uncommon in the United States; it is not a reportable disease, and the prevalence is unknown Patel, et al. 2019. The majority of cases occur among people who migrate or travel to the United States from countries with high HDV endemicity (i.e., Eastern Europe, Southern Europe, the Mediterranean region, the Middle East, West and Central Africa, East Asia, and the Amazon River Basin in South America) CDC(a) 2020.
Existing data indicate that pegylated interferon (PEG-IFN) is the only effective anti-HDV treatment EASL 2012. However, fewer than 30% of people without HIV who have HDV achieve sustained HDV suppression when treated with PEG-IFN Wedemeyer et al. 2011. No data are available regarding the efficacy of PEG-IFN therapy in patients with HIV/HBV/HDV tri-infection. Because HDV depends on HBV to replicate, HBsAg seroconversion should be the primary goal for patients with HIV/HBV/HDV tri-infection. In patients with tri-infection, prompt initiation of anti-HBV and anti-HIV therapy should be strongly encouraged.
Little guidance is available on optimal monitoring strategies for patients with HIV/HBV coinfection and positive serum anti-HDV total (IgM and IgG) test results. It is reasonable to perform baseline HDV RNA testing and consult with an experienced care provider about ongoing HDV RNA and DNA testing Farci and Niro 2018.
HBV Treatment and Monitoring
| RECOMMENDATIONS |
Treatment
Pregnant Patients
Monitoring
|
Abbreviations: 3TC, lamivudine; ALT, alanine transaminase; ART, antiretroviral therapy; ETV, entecavir; FTC, emtricitabine; HBV, hepatitis B virus; HDV, hepatitis D virus; IRIS, immune reconstitution inflammatory syndrome; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. |
Treatment
Goals: The goals of treatment for chronic HBV infection in adults with HIV are to reduce liver inflammation (as indicated by normalization of ALT), obtain seroconversion of hepatitis B e antigen (HBeAg) to antibody to HBeAg, and suppress HBV viral replication. These changes will help reduce the risk of hepatic decompensation, halt or reverse liver fibrosis, prevent the development of hepatocellular carcinoma (HCC), and decrease HBV-related mortality Kim, et al. 2021; Terrault, et al. 2018; Soriano, et al. 2008.
As indicated in the NYSDOH AI guideline Rapid ART Initiation, clinicians should recommend ART to all patients diagnosed with HIV infection. For patients with HIV/HBV coinfection, the regimen should include medications that suppress both HIV and HBV (see Table 4, below). Optimal treatment for both viruses should be taken simultaneously to prevent the development of HIV and HBV drug resistance. Optimal treatment of both infections may also help reduce the risk of IRIS, which is increased in patients with high levels of HBV viremia (see guideline section Monitoring, below) Avihingsanon, et al. 2012; Crane, et al. 2009.
| Abbreviations: ART, antiretroviral therapy; CrCl, creatinine clearance; HBV, hepatitis B virus; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PEG-IFN, pegylated interferon. | |
| Table 4: Available Medications for Treatment of HBV Infection in Adults With HIV | |
| Medication | Clinical Comment |
| Tenofovir disoproxil fumarate (TDF) |
|
| Tenofovir alafenamide (TAF) |
|
| Lamivudine (3TC) |
|
| Emtricitabine (FTC) |
|
| Entecavir (ETV) |
|
| Interferon (IFN) |
|
Preferred regimen: Because FTC, 3TC, TDF, and TAF all have activity against HIV and HBV, an ART regimen for a patient with HIV/HBV coinfection should include a nucleoside/nucleotide reverse transcriptase inhibitor backbone of either TAF/FTC, TDF/FTC, or TDF/3TC as part of a fully suppressive regimen. TDF or TAF should not be used alone in the absence of a fully suppressive ART regimen because resistance mutations may develop DHHS 2022; DHHS 2019. For the use of TDF or TAF in patients with reduced renal function, see the NYSDOH AI guideline Selecting an Initial ART Regimen > ARV Dose Adjustments for Hepatic or Renal Impairment.
Alternative regimen: If patients cannot or choose not to take TDF or TAF, the alternative recommended regimen is ETV in addition to a fully suppressive HIV ART regimen DHHS 2022; DHHS 2019. ETV should not be considered part of the HIV ART regimen. The ETV dose should be increased from 0.5 mg per day to 1.0 mg per day in patients with known or suspected 3TC-resistant HBV infection. However, ETV resistance may emerge rapidly in patients with 3TC-resistant HBV infection Terrault, et al. 2018. Therefore, ETV should be used with caution in patients with HIV/HBV coinfection who do not take TAF or TDF, and frequent monitoring (every 3 months) of HBV DNA levels should be performed to detect viral breakthrough (see guideline section Monitoring, below).
The anti-HBV activity of 3TC, FTC, TDF, and TAF warrants their continued use whenever possible, even when HIV resistance indicates that they should be discontinued as part of the ART regimen. These agents should be continued after an anti-HBV therapy response has been achieved, even if the ART regimen has to be changed. Patients should be advised against discontinuing HIV or HBV treatment because the cessation of therapy has been associated with reactivation of HBV leading to exacerbations of hepatitis and hepatic failure DHHS 2022. Hepatitis flares can occur in patients with HBV monoinfection and those with HIV/HBV coinfection, but the risk of hepatic injury and fulminant hepatic failure is greater in patients with HIV/HBV coinfection Moreno-Cubero, et al. 2018; Boyd, et al. 2017; Dore, et al. 2010.
Two-drug regimens for HIV: For patients with HIV/HBV coinfection, a 2-drug ART regimen should not be used as initial ART unless combined with an additional agent(s) with activity against HBV (see Table 5, below, for recommended additions). The same is true for patients with controlled HIV/HBV coinfection who switch to a 2-drug regimen for HIV ART—an agent with anti-HBV activity is required. Patients switching to a 2-drug regimen for HIV plus the additional agent(s) to treat HBV should be closely monitored for potential HBV flare (see guideline section Monitoring, below).
| Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; CAB, cabotegravir; DTG, dolutegravir; ETV, entecavir; FTC, emtricitabine; HBV, hepatitis B virus; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. | |
| Table 5: Recommended Additions to 2-Drug HIV ART Regimens for Patients With Chronic HBV | |
| Drug HIV ART Regimen | Addition for HBV Treatment |
| DTG/3TC | TAF, TDF, or ETV |
| DTG/RPV | TAF/FTC, TDF/FTC, or TDF/3TC |
| CAB/RPV | TAF/FTC, TDF/FTC, or TDF/3TC |
HBV treatment during pregnancy: Clinicians should offer pregnant patients with HIV/HBV coinfection ART that includes agents active against HIV and HBV. 3TC, FTC, TAF, and TDF can be safely used during pregnancy DHHS 2022; Terrault, et al. 2018. The preferred regimen is DTG plus TDF or TAF in combination with either FTC or 3TC. An alternative regimen is ritonavir-boosted darunavir (DRV/r) plus TDF or TAF with FTC or 3TC DHHS 2022.
Monitoring
Recommended clinical evaluation and laboratory monitoring are described in Table 6, below.
| Abbreviations: HBeAg, hepatitis B e antigen; HBV, hepatitis B virus.
Notes:
|
|||
| Table 6: Recommended Monitoring After HBV Treatment Initiation in Adults With HIV | |||
| Laboratory Test | Every 3 Months | Every 6 Months | Every 12 Months |
| HBV DNA | Until HBV DNA is undetectable [a] | After HBV DNA is undetectable | |
| HBeAg | Check for HBeAg-negative result [b] | ||
| Electrolyte panel | X | ||
| Serum creatinine | X | ||
| Urinalysis [c] | X | ||
| Liver function panel [c] | Until HBV DNA is undetectable [a] | After HBV DNA is undetectable | |
Response to treatment: HBV viral load levels generally decline more slowly after treatment initiation than HIV viral load levels. Anti-HBV treatment responses are defined as follows DHHS 2022:
- Primary nonresponse: HBV DNA <1 log10 decline at 12 weeks
- Complete virologic response: Undetectable HBV DNA by polymerase chain reaction assay at 24 to 48 weeks
- Partial virologic response: ≥1 log10 decline but still detectable HBV DNA at 24 weeks
- Maintained virologic response: A response that continues while on therapy
- Sustained virologic response: A virologic response that is still present 6 months after cessation of therapy
Renal toxicity: Renal toxicity with increased creatinine or renal tubular dysfunction has been associated with tenofovir use, and the association is stronger with TDF than TAF Gupta, et al. 2019. This renal toxicity may be reversible with dose adjustments of TDF or switching to TAF. Clinicians should evaluate electrolytes, serum creatinine levels, and urinalysis every 6 months DHHS 2022.
Cirrhosis: Patients with HIV/HBV coinfection and cirrhosis should be referred to a gastroenterologist or hepatologist to assess and manage complications of portal hypertension such as gastroesophageal varices and ascites. Patients with HIV/HBV coinfection and cirrhosis should undergo esophagogastroduodenoscopy at the time of chronic HBV diagnosis and every 1 to 2 years thereafter DHHS 2022; Terrault, et al. 2018.
Acute flare: If a patient being treated for chronic HBV develops signs or symptoms of acute hepatitis (nausea, vomiting, elevated ALT or bilirubin levels), clinicians should evaluate the patient, rule out HBV IRIS and HDV flare among other potential causes, and consult with an HIV-experienced hepatologist. Hepatic flares are usually mild and self-limited but can result in decompensation in individuals with preexisting cirrhosis Anderson, et al. 2010; Crane, et al. 2009; Perrella, et al. 2006; Konopnicki, et al. 2005; Drake, et al. 2004.
In patients with HIV, initiation of or a change in ART introduces the potential for IRIS, which may manifest as a worsening of previously diagnosed disease or the appearance of a previously undiagnosed disease. In patients with HIV/HBV coinfection, IRIS can present as an acute flare of HBV disease. It can often be difficult to distinguish HBV IRIS from other causes of an acute HBV flare, such as drug or alcohol hepatotoxicity or other viral infection (hepatitis A, C, D, or E virus, Epstein-Barr virus, herpes simplex virus, or cytomegalovirus). Reviewing medication history and testing for serum HBV DNA, HBeAg, HIV viral load, and CD4 cell count can help distinguish between these possibilities DHHS 2022.
HBV IRIS is usually detected within the first 6 to 12 weeks after ART is initiated, based on a noticeable rise in ALT levels that coincides with rising CD4 cell counts (immune reconstitution) and signs and symptoms characteristic of acute hepatitis and with no other cause for the flare DHHS 2022. Risk factors for HBV IRIS include high HBV viral load, elevated ALT level, and low CD4 cell count at baseline Singh, et al. 2017.
Ongoing Screening for Hepatocellular Carcinoma
Compared with HBV monoinfection, HIV/HBV coinfection is associated with an increased risk of developing HCC and increased mortality rates Sun, et al. 2021; Pinato, et al. 2019; Singh, et al. 2017. Patients with HIV/HBV coinfection and cirrhosis should be screened with ultrasound for HCC every 6 months Terrault, et al. 2018.
There is no consensus on how frequently to screen for HCC in patients with HIV/HBV coinfection who do not have cirrhosis. In patients with HBV monoinfection, screening is recommended every 6 months for groups at increased risk for developing HCC, including Asian men >40 years old, Asian women >50 years old, Black men >40 years old, individuals with a first-degree family member who has a history of HCC, or individuals with HDV Terrault, et al. 2018; Sarin, et al. 2016; Zhang, et al. 2004.
All Recommendations
| ALL RECOMMENDATIONS: PREVENTION AND MANAGEMENT OF HEPATITIS B VIRUS INFECTION IN ADULTS WITH HIV |
Screening Tests
Diagnosis
Acute HBV Infection
Transmission Prevention
Primary Vaccination
Revaccination
Liver Disease Assessment
Alcohol Use Screening and Education
HAV, HCV, and HDV Status
Treatment
Pregnant Patients
Monitoring
|
Abbreviations: 3TC, lamivudine; ALT, alanine transaminase; anti-HBc, hepatitis B core antibody; anti-HBe, antibody to HBeAg; anti-HBs, hepatitis B surface antibody; ART, antiretroviral therapy; AST, aspartate transaminase; CBC, complete blood count; DAA, direct-acting antiviral; ETV, entecavir; FTC, emtricitabine; HAV, hepatitis A virus; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; IgG, immunoglobulin G; IgM, immunoglobulin M; IRIS, immune reconstitution inflammatory syndrome; PT/INR, prothrombin time/international normalized ratio; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate. Note:
|
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
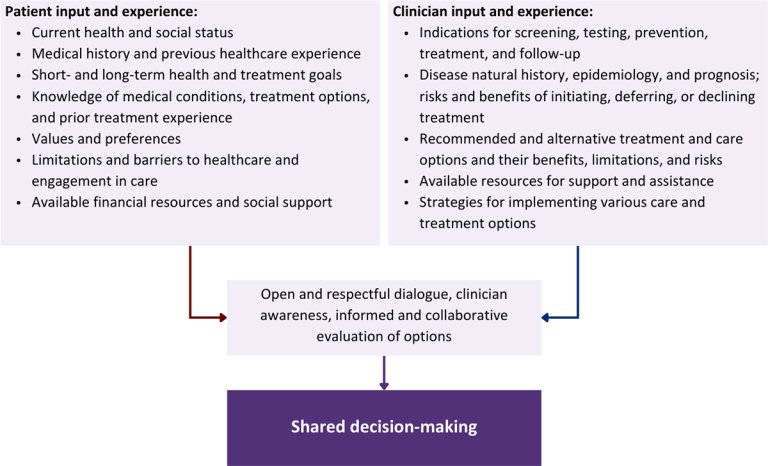
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making
Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Agarwal K., Brunetto M., Seto W. K., et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol 2018;68(4):672-81. [PMID: 29756595]
Alter M. J. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006;44(1 Suppl):S6-9. [PMID: 16352363]
Anderson A. M., Mosunjac M. B., Palmore M. P., et al. Development of fatal acute liver failure in HIV-HBV coinfected patients. World J Gastroenterol 2010;16(32):4107-11. [PMID: 20731028]
Avihingsanon A., Matthews G. V., Lewin S. R., et al. Assessment of HBV flare in a randomized clinical trial in HIV/HBV coinfected subjects initiating HBV-active antiretroviral therapy in Thailand. AIDS Res Ther 2012;9(1):6. [PMID: 22405335]
Awad A. M., Ntoso A., Connaire J. J., et al. An open-label, single-arm study evaluating the immunogenicity and safety of the hepatitis B vaccine HepB-CpG (HEPLISAV-B®) in adults receiving hemodialysis. Vaccine 2021;39(25):3346-52. [PMID: 34001345]
Béguelin C., Moradpour D., Sahli R., et al. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol 2017;66(2):297-303. [PMID: 27746337]
Benhamou Y., Bochet M., Thibault V., et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999;30(5):1302-6. [PMID: 10534354]
Bennett J., Dolin R., Blaser M. J. Mandell, Douglas, and Bennett's principles and practice of infectious diseases; 2019. https://www.elsevier.com/books/mandell-douglas-and-bennetts-principles-and-practice-of-infectious-diseases/bennett/978-0-323-48255-4
Bhattacharya D., Tseng C. H., Tate J. P., et al. Isolated hepatitis B core antibody is associated with advanced hepatic fibrosis in HIV/HCV infection but not in HIV infection alone. J Acquir Immune Defic Syndr 2016;72(1):e14-7. [PMID: 26829660]
Boyd A., Houghtaling L., Moh R., et al. Clinical outcomes during treatment interruptions in human immunodeficiency virus-hepatitis B virus co-infected patients from Sub-Saharan Africa. Am J Trop Med Hyg 2017;97(6):1936-42. [PMID: 29141712]
Bräu N., Fox R. K., Xiao P., et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol 2007;47(4):527-37. [PMID: 17692986]
Callebaut C., Stepan G., Tian Y., et al. In vitro virology profile of tenofovir alafenamide, a novel oral prodrug of tenofovir with improved antiviral activity compared to that of tenofovir disoproxil fumarate. Antimicrob Agents Chemother 2015;59(10):5909-16. [PMID: 26149992]
Castellares C., Barreiro P., Martín-Carbonero L., et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat 2008;15(3):165-72. [PMID: 18233989]
CDC(a). Viral hepatitis: hepatitis D. 2020 Jun 22. https://www.cdc.gov/hepatitis/hdv/index.htm [accessed 2022 Jul 18]
CDC(a). National Center for Health Statistics: alcohol use. 2022 Jan 13. https://www.cdc.gov/nchs/fastats/alcohol.htm [accessed 2022 Jul 18]
CDC(b). Viral hepatitis surveillance report 2018 — hepatitis B. 2020 Jul 27. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepB.htm#Figure2.8 [accessed 2022 Jul 15]
CDC(b). National Center for Health Statistics: chronic liver disease and chirrhosis. 2022 Jan 5. https://www.cdc.gov/nchs/fastats/liver-disease.htm [accessed 2022 Mar 21]
Chaiklang K., Wipasa J., Chaiwarith R., et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8(11):e80409. [PMID: 24265819]
Chang J. J., Mohtashemi N., Bhattacharya D. Significance and management of isolated hepatitis B core antibody (anti-HBc) in HIV and HCV: strategies in the DAA era. Curr HIV/AIDS Rep 2018;15(2):172-81. [PMID: 29572624]
Crane M., Oliver B., Matthews G., et al. Immunopathogenesis of hepatic flare in HIV/hepatitis B virus (HBV)-coinfected individuals after the initiation of HBV-active antiretroviral therapy. J Infect Dis 2009;199(7):974-81. [PMID: 19231993]
de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63(3):743-52. [PMID: 26047908]
de Vries-Sluijs T. E., Hansen B. E., van Doornum G. J., et al. A randomized controlled study of accelerated versus standard hepatitis B vaccination in HIV-positive patients. J Infect Dis 2011;203(7):984-91. [PMID: 21266513]
de Vries-Sluijs T. E., Hansen B. E., van Doornum G. J., et al. A prospective open study of the efficacy of high-dose recombinant hepatitis B rechallenge vaccination in HIV-infected patients. J Infect Dis 2008;197(2):292-94. [PMID: 18177248]
DHHS. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2019 Dec 18. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/initiation-antiretroviral-therapy?view=full [accessed 2022 Jul 18]
DHHS. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. 2022 Nov 13. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/hepatitis-b-virus-infection?view=full [accessed 2021 Apr 16]
Donato F., Tagger A., Chiesa R., et al. Hepatitis B and C virus infection, alcohol drinking, and hepatocellular carcinoma: a case-control study in Italy. Brescia HCC Study. Hepatology 1997;26(3):579-84. [PMID: 9303486]
Dore G. J., Soriano V., Rockstroh J., et al. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 2010;24(6):857-65. [PMID: 20216301]
Drake A., Mijch A., Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39(1):129-32. [PMID: 15206064]
EASL. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57(1):167-85. [PMID: 22436845]
Farci P., Niro G. A. Current and future management of chronic hepatitis D. Gastroenterol Hepatol (N Y) 2018;14(6):342-51. [PMID: 30166948]
FDA. Heplisav-B [hepatitis B vaccine (recombinant), adjuvanted] solution for intramuscular injection. 2020 May. https://www.fda.gov/vaccines-blood-biologics/vaccines/heplisav-b [accessed 2022 Jul 18]
FDA. Prehevbrio [hepatitis B vaccine (recombinant)]. 2021 Dec 1. https://www.fda.gov/media/154561/download [accessed 2022 Mar 21]
FDA(a). Engerix-B [hepatitis B vaccine (recombinant)] injectable suspension, for instramuscular use. 2018 Dec 21. https://www.fda.gov/media/119403/download [accessed 2022 Mar 2]
FDA(b). Recombivax HB hepatitis B vaccine (recombinant). 2018 Dec. https://www.fda.gov/media/74274/download [accessed 2022 Mar 2]
FDA(c). Twinrix [hepatitis A & hepatitis B (recombinant) vaccine] injectable suspension, for intramuscular use. 2018 Dec 18. https://www.fda.gov/media/119351/download [accessed 2022 Mar 2]
Fernández-Montero J. V., Vispo E., Barreiro P., et al. Hepatitis delta is a major determinant of liver decompensation events and death in HIV-infected patients. Clin Infect Dis 2014;58(11):1549-53. [PMID: 24633686]
Fonseca M. O., Pang L. W., de Paula Cavalheiro N., et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23(22):2902-8. [PMID: 15780739]
Gallant J. The M184V mutation: what it does, how to prevent it, and what to do with it when it's there. AIDS Read 2006;16(10):556-9. [PMID: 17096474]
Gallant J., Brunetta J., Crofoot G., et al. Brief report: efficacy and safety of switching to a single-tablet regimen of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide in HIV-1/hepatitis B-coinfected adults. J Acquir Immune Defic Syndr 2016;73(3):294-98. [PMID: 27171740]
Gandhi R. T., Wurcel A., Lee H., et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191(9):1435-41. [PMID: 15809901]
Garcia-Tsao G., Abraldes J. G., Berzigotti A., et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65(1):310-35. [PMID: 27786365]
Gupta S. K. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS 2008;22(2):99-103. [PMID: 18260800]
Gupta S. K., Post F. A., Arribas J. R., et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS 2019;33(9):1455-65. [PMID: 30932951]
Keet I. P., van Doornum G., Safary A., et al. Insufficient response to hepatitis B vaccination in HIV-positive homosexual men. AIDS 1992;6(5):509-10. [PMID: 1535502]
Khaimova R., Fischetti B., Cope R., et al. Serological response with Heplisav-B® in prior hepatitis B vaccine non-responders living with HIV. Vaccine 2021;39(44):6529-34. [PMID: 34600748]
Kim H. N., Newcomb C. W., Carbonari D. M., et al. Risk of HCC with hepatitis B viremia among HIV/HBV-coinfected persons in North America. Hepatology 2021;74(3):1190-1202. [PMID: 33780007]
Knox T. A., Jerger L., Tang A. M. Alcohol, nutrition, and health consequences: alcohol, HIV/AIDS, and liver disease; 2013. https://link.springer.com/chapter/10.1007/978-1-62703-047-2_23
Komatsu H., Inui A., Sogo T., et al. Tears from children with chronic hepatitis B virus (HBV) infection are infectious vehicles of HBV transmission: experimental transmission of HBV by tears, using mice with chimeric human livers. J Infect Dis 2012;206(4):478-85. [PMID: 22508939]
Konopnicki D., Mocroft A., de Wit S., et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19(6):593-601. [PMID: 15802978]
Lampertico P., Buti M., Fung S., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: a randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol Hepatol 2020;5(5):441-53. [PMID: 32087795]
Launay O., van der Vliet D., Rosenberg A. R., et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305(14):1432-40. [PMID: 21486976]
Lim J. K., Nguyen M. H., Kim W. R., et al. Prevalence of chronic hepatitis B virus infection in the United States. Am J Gastroenterol 2020;115(9):1429-38. [PMID: 32483003]
Lok A. S., Lai C. L., Wu P. C. Prevalence of isolated antibody to hepatitis B core antigen in an area endemic for hepatitis B virus infection: implications in hepatitis B vaccination programs. Hepatology 1988;8(4):766-70. [PMID: 2968945]
Loke R. H., Murray-Lyon I. M., Coleman J. C., et al. Diminished response to recombinant hepatitis B vaccine in homosexual men with HIV antibody: an indicator of poor prognosis. J Med Virol 1990;31(2):109-11. [PMID: 2143776]
Marcellin P., Pequignot F., Delarocque-Astagneau E., et al. Mortality related to chronic hepatitis B and chronic hepatitis C in France: evidence for the role of HIV coinfection and alcohol consumption. J Hepatol 2008;48(2):200-207. [PMID: 18086507]
Mast E. E., Weinbaum C. M., Fiore A. E., et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults. MMWR Recomm Rep 2006;55(Rr-16):1-33; quiz CE1-4. [PMID: 17159833]
Mavilia M. G., Wu G. Y. HBV-HCV coinfection: viral interactions, management, and viral reactivation. J Clin Transl Hepatol 2018;6(3):296-305. [PMID: 30271742]
McComsey G. A., Kitch D., Daar E. S., et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011;203(12):1791-1801. [PMID: 21606537]
Moreno-Cubero E., Del Arco R. T., Peña-Asensio J., et al. Is it possible to stop nucleos(t)ide analogue treatment in chronic hepatitis B patients?. World J Gastroenterol 2018;24(17):1825-38. [PMID: 29740199]
Nakanuma Y., Ohta G. Morphology of cirrhosis and occurrence of hepatocellular carcinoma in alcoholics with and without HBsAg and in non-alcoholic HBsAg-positive patients. A comparative autopsy study. Liver 1983;3(4):231-37. [PMID: 6323910]
Neau D., Winnock M., Jouvencel A. C., et al. Occult hepatitis B virus infection in HIV-infected patients with isolated antibodies to hepatitis B core antigen: Aquitaine cohort, 2002-2003. Clin Infect Dis 2005;40(5):750-53. [PMID: 15714424]
Ni J. D., Xiong Y. Z., Wang X. J., et al. Does increased hepatitis B vaccination dose lead to a better immune response in HIV-infected patients than standard dose vaccination: a meta-analysis?. Int J STD AIDS 2013;24(2):117-22. [PMID: 23467291]
Núñez M., Lana R., Mendoza J. L., et al. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001;27(5):426-31. [PMID: 11511818]
Overton E. T., Sungkanuparph S., Powderly W. G., et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41(7):1045-48. [PMID: 16142673]
Patel E. U., Thio C. L., Boon D., et al. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011-2016. Clin Infect Dis 2019;69(4):709-12. [PMID: 30605508]
Perrella O., Sbreglia C., De Sena R., et al. Immune reconstitution: bad or good factor in hepatitis B virus and HIV co-infection?. AIDS 2006;20(5):790-91. [PMID: 16514319]
Pinato D. J., Allara E., Chen T. Y., et al. Influence of HIV infection on the natural history of hepatocellular carcinoma: results from a global multicohort study. J Clin Oncol 2019;37(4):296-304. [PMID: 30562130]
Piroth L., Launay O., Michel M. L., et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213(11):1735-42. [PMID: 26768256]
Poynard T., Mathurin P., Lai C. L., et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol 2003;38(3):257-65. [PMID: 12586290]
Psevdos G., Kim J. H., Groce V., et al. Efficacy of double-dose hepatitis B rescue vaccination in HIV-infected patients. AIDS Patient Care STDS 2010;24(7):403-7. [PMID: 20586648]
Raimondo G., Pollicino T., Cacciola I., et al. Occult hepatitis B virus infection. J Hepatol 2007;46(1):160-70. [PMID: 17112622]
Rey D., Krantz V., Partisani M., et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18(13):1161-65. [PMID: 10649616]
Rey D., Piroth L., Wendling M. J., et al. Safety and immunogenicity of double-dose versus standard-dose hepatitis B revaccination in non-responding adults with HIV-1 (ANRS HB04 B-BOOST): a multicentre, open-label, randomised controlled trial. Lancet Infect Dis 2015;15(11):1283-91. [PMID: 26257021]
Rosenthal E. M., Hall E. W., Rosenberg E. S., et al. Assessing the cost-utility of preferentially administering Heplisav-B vaccine to certain populations. Vaccine 2020;38(51):8206-15. [PMID: 33160756]
Rubin L. G., Levin M. J., Ljungman P., et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58(3):e44-100. [PMID: 24311479]
Sarin S. K., Kumar M., Lau G. K., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10(1):1-98. [PMID: 26563120]
Schnittman S. R., Zepf R., Cocohoba J., et al. Brief report: Heplisav-B seroprotection in people with HIV: a single-center experience. J Acquir Immune Defic Syndr 2021;86(4):445-49. [PMID: 33196553]
Sheng W. H., Hung C. C., Kao J. H., et al. Impact of hepatitis D virus infection on the long-term outcomes of patients with hepatitis B virus and HIV coinfection in the era of highly active antiretroviral therapy: a matched cohort study. Clin Infect Dis 2007;44(7):988-95. [PMID: 17342655]
Shiffman M. L. Management of acute hepatitis B. Clin Liver Dis 2010;14(1):75-91; viii-ix. [PMID: 20123442]
Singh K. P., Crane M., Audsley J., et al. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 2017;31(15):2035-52. [PMID: 28692539]
Smith D. K., Herbst J. H., Zhang X., et al. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2015;68(3):337-44. [PMID: 25469526]
Soriano V., Puoti M., Peters M., et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008;22(12):1399-1410. [PMID: 18614862]
Spradling P. R., Richardson J. T., Buchacz K., et al. Prevalence of chronic hepatitis B virus infection among patients in the HIV Outpatient Study, 1996-2007. J Viral Hepat 2010;17(12):879-86. [PMID: 20158604]
StatPearls. Hepatitis B. 2022 Jun 11. https://www.ncbi.nlm.nih.gov/books/NBK555945/ [accessed 2022 Jul 18]
Sun J., Althoff K. N., Jing Y., et al. Trends in hepatocellular carcinoma incidence and risk among persons with HIV in the US and Canada, 1996-2015. JAMA Netw Open 2021;4(2):e2037512. [PMID: 33595662]
Tayal S. C., Sankar K. N. Impaired response to recombinant hepatitis B vaccine in asymptomatic HIV-infected individuals. AIDS 1994;8(4):558-59. [PMID: 7912087]
Tedaldi E. M., Baker R. K., Moorman A. C., et al. Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis 2004;38(10):1478-84. [PMID: 15156488]
Terrault N. A., Lok A. S., McMahon B. J., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67(4):1560-99. [PMID: 29405329]
Thio C. L. Hepatitis B and human immunodeficiency virus coinfection. Hepatology 2009;49(5 Suppl):S138-45. [PMID: 19399813]
Thio C. L., Seaberg E. C., Skolasky R., et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921-26. [PMID: 12493258]
Tran T. T. Immune tolerant hepatitis B: a clinical dilemma. Gastroenterol Hepatol (N Y) 2011;7(8):511-16. [PMID: 22298987]
Veiga A. P., Casseb J., Duarte A. J. Humoral response to hepatitis B vaccination and its relationship with T CD45RA+ (naïve) and CD45RO+ (memory) subsets in HIV-1-infected subjects. Vaccine 2006;24(49-50):7124-28. [PMID: 16884833]
Velasquez M. M., von Sternberg K., Johnson D. H., et al. Reducing sexual risk behaviors and alcohol use among HIV-positive men who have sex with men: a randomized clinical trial. J Consult Clin Psychol 2009;77(4):657-67. [PMID: 19634959]
Wedemeyer H., Yurdaydìn C., Dalekos G. N., et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364(4):322-31. [PMID: 21268724]
Weller S., Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev 2002;(1):CD003255. [PMID: 11869658]
Witt M. D., Lewis R. J., Rieg G., et al. Predictors of the isolated hepatitis B core antibody pattern in HIV-infected and -uninfected men in the Multicenter AIDS Cohort Study. Clin Infect Dis 2013;56(4):606-12. [PMID: 23090927]
Zhang B. H., Yang B. H., Tang Z. Y. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130(7):417-22. [PMID: 15042359]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | August 01, 2013 |
| Date of current publication | August 30, 2022 |
| Highlights of changes, additions, and updates in the August 30, 2022 edition |
Comprehensive update |
| Intended users | New York State clinicians in outpatient settings who provide primary and HIV specialty care for adults who have or are at risk of acquiring hepatitis B virus infection |
| Lead author |
Alok Gupta, MBBS |
| Writing group |
Steven M. Fine, MD, PhD; Rona M. Vail, MD; Joseph P. McGowan, MD, FACP, FIDSA; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD, FACP, AAHIVS; Charles J. Gonzalez, MD; Christopher J. Hoffmann, MD, MPH |
| Author and writing group conflict of interest disclosures |
Joseph P. McGowan, MD, FACP, FIDSA: Institutional Pharma grant recipient/support, clinical trial; Gilead |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines |
|
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on April 1, 2024

