Purpose of This Guideline
Date of current publication: March 25, 2022
Lead authors: Maria Teresa (Tess) Timoney, MS, RN, CNM; Jessica M. Atrio, MD, MSc
Writing group: Joseph P. McGowan, MD, FACP, FIDSA; Steven M. Fine, MD, PhD; Rona Vail, MD; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: November 15, 2017
Purpose: This guideline on cervical cancer screening for adults with HIV was developed by the New York State Department of Health AIDS Institute (NYSDOH AI) to inform primary care providers and other practitioners in New York State about screening for cervical dysplasia in patients with HIV. The goal of cervical screening is to identify and treat precancerous lesions to prevent cervical cancer. Comprehensive primary care for adults with HIV includes access to antiretroviral therapy (ART) and screening, diagnosis, and treatment of gynecologic comorbidities, especially cervical dysplasia and cancer.
Screening for cervical and anogenital tract cancer is appropriate for all adult patients; this guideline provides standards of care for cervical, vaginal, and genital screening for patients with HIV. Inclusive and culturally sensitive healthcare that acknowledges the needs of transgender, transmasculine, transfeminine, and nonbinary patients should include an anatomical inventory that identifies which organs are present and absent to determine and meet the screening and healthcare needs of each patient regardless of their gender expression.
Goals: This guideline addresses the prevention of, screening methods for, and diagnosis of genital dysplasia in patients with HIV to achieve the following:
- Increase the number of New York State residents with HIV who are screened for and receive effective medical management of cervical, vaginal, or vulvar dysplasia.
- Emphasize the role of ART-associated viral suppression in improving clearance or suppression of human papillomavirus (HPV), preventing cervical dysplasia, and reducing cervical cancer in individuals with HIV.
- Reduce the incident morbidity and mortality associated with genital HPV disease in individuals with HIV through vaccination against HPV and identification and treatment of precancerous lesions, when treatment is most successful, and cancerous lesions.
- Support the NYSDOH Prevention Agenda 2019-2024, which aims to increase cervical cancer screening by 5% among individuals who are 21 to 65 years old and have an annual income below $25,000.
- Integrate current evidence-based clinical recommendations into the healthcare-related implementation strategies of the New York State Ending the Epidemic initiative.
HPV-Associated Cervical Disease
The American Cancer Society estimates that in the United States in 2022, approximately 14,100 new cases of invasive cervical cancer will be diagnosed, and approximately 4,280 individuals will die from cervical cancer ACS 2022. In 2018, there were 792 new cases of cervical cancer among all women in New York State, with 233 deaths from the disease CDC(d) 2021. Nearly 100% of cases of cervical cancer are associated with HPV infection CDC(a) 2021; CDC(b) 2021; Chaturvedi, et al. 2011; Winer, et al. 2006. Individuals with HIV are at increased risk of human papillomavirus (HPV) infection and related disease and are 5 times more likely than those without HIV to be diagnosed with cervical cancer Liu, et al. 2018; Grulich, et al. 2007. Cervical cancer is an AIDS-defining illness.
In the general population, the HPV subtypes most commonly associated with cervical cancer are 16 and 18 CDC(a) 2021, and infection with multiple HPV subtypes has been associated with benign condylomata acuminata (genital warts), squamous intraepithelial lesions (SILs), vulvar and anal dysplasia, and anogenital carcinoma NCI SEER 2018. In individuals with HIV, a broader range of HPV oncogenic subtypes are associated with cervical dysplasia Orlando, et al. 2017.
HPV-related cervical cell abnormalities: Persistent HPV infection is necessary for the development of cervical SILs, which arise at the junction of the cervical squamous and columnar epithelium around the cervical os, the transformation zone. SILs are the most common type of precancerous cervical lesions, preceding nearly 80% of cervical cancers ICESCC 2007. Glandular carcinomas are the second most common type of cervical cancer ICESCC 2007. SILs occur more frequently in individuals with HIV than in those without HIV Liu, et al. 2018; Maiman, et al. 1993.
Cervical cell abnormalities can be categorized as high risk (cancer causing) or low risk (benign warts) based on oncogenic potential. High-risk HPV types that are related to anogenital cancers include types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, and 82 Guan, et al. 2012; Hariri, et al. 2012. Although high-risk HPV types are detected in 99% of cervical cancers, types 16 and 18 are the most oncogenic Clifford, et al. 2017; Keller, et al. 2015 and account for nearly 70% of all cervical cancers in the general population CDC(a) 2021.
Among individuals with HIV, cancer is associated with types 16 and 18 and high-risk types 51, 52, 53, 56, 58, and 59 McKenzie, et al. 2010, and low-risk types 6 and 11 are most commonly associated with benign disease (genital warts) McKenzie, et al. 2010. Identifying the presence of high-risk HPV types is central to managing abnormal cytology results in individuals with and without HIV Perkins, et al. 2020; Hariri, et al. 2012.
Higher risk of cervical disease associated with HIV: The risk of HPV-related cervical disease is increased in individuals with HIV McClymont, et al. 2020; Grabar, et al. 2019; Liu, et al. 2018; Konopnicki, et al. 2013. Cervical cancer has historically been a leading cause of cancer death among individuals with HIV Dryden-Peterson, et al. 2016, which may be related to the increased prevalence and persistence of HPV in this population Kojic, et al. 2014; Moscicki, et al. 2004.
HPV Prevention
| RECOMMENDATION |
HPV Prevention
|
HPV Vaccine
In 2006, the U.S. Food and Drug Administration (FDA) approved a 9-valent vaccine that protects against nononcogenic HPV types 6 and 11 and oncogenic HPV types 16, 18, 31, 33, 45, 52, and 58 (Gardasil 9). Because it offers broader coverage of HPV types than other approved bivalent vaccines, the 9-valent vaccine is the only HPV vaccine available in the United States (see CDC: Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV for more information). The HPV vaccine is approved by the FDA for preventive but not therapeutic use.
Extrapolating data from the demonstrated effectiveness of the quadrivalent HPV vaccine in older individuals Wilkin, et al. 2018, the FDA expanded the age range for approved use of the HPV vaccine in the United States from ages 9 to 26 years to ages 9 to 45 years FDA 2020. There is no specific mention of HIV infection in the updated FDA approval. Although 1 study demonstrated lower efficacy of the quadrivalent vaccine in individuals with HIV Wilkin, et al. 2018, other research has linked HIV viral suppression to vaccine efficacy Money, et al. 2016. Given the increased lifetime burden of persistent HPV infection, disease, and morbidity, proactive vaccination among individuals with HIV is a strategic means of primary prevention and potential disease mitigation that should be strongly considered and encouraged Di Donato, et al. 2021; Karimi-Zarchi, et al. 2020; Lichter, et al. 2020.
When to Vaccinate
HPV vaccination for all individuals may be scheduled at the same time as standard adolescent vaccines offered at ages 9 to 12 years. If possible, the HPV vaccine series should begin at age 9 years Glidden, et al. 2016. The 3-dose vaccine is recommended for all patients with HIV who are 9 to 45 years old. The 9-valent HPV vaccine should be administered according to the CDC standard schedule for immunocompromised adults, children, and adolescents (a 3-dose regimen over a 6-month period at 0, 2, and 6 months) and should be offered regardless of CD4 cell count.
HPV vaccination provides high levels of neutralizing antibodies for at least 5 years and is protective in individuals ≤26 years old who do not have HIV, regardless of history of sexual activity; however, the full length of its protection has not been established. In an observational study conducted in England that examined the effectiveness of a national HPV immunization program, the reduction in cervical cancer was greatest in individuals who received the vaccine at ages 12 to 13 years Falcaro, et al. 2021. Although data are limited, the immunogenicity of the quadrivalent HPV vaccine has been demonstrated in individuals with HIV Wilkin, et al. 2018; Kojic, et al. 2014.
HPV testing and vaccination: HPV testing is not recommended before vaccine administration. It is unlikely that an individual will have been infected with all the HPV types covered by the 9-valent vaccine; therefore, it is expected that the 9-valent HPV vaccine will be effective against any of the 9 HPV types or any HPV types to which the individual has not yet been exposed. There also may be beneficial prevention due to cross-reactivity with other HPV types not included in the 9-valent vaccine Wheeler, et al. 2012.
Revaccination with the 9-valent HPV vaccine is not currently recommended for individuals previously immunized with the bivalent or quadrivalent HPV vaccine ACOG 2020; Petrosky, et al. 2015. Vaccination with the quadrivalent HPV vaccine has demonstrated cross-protection against other oncogenic HPV types Kemp, et al. 2011. There is no maximum interval between vaccine doses; as long as all 3 doses are given, there is no need to repeat doses if a scheduled vaccination is not given on schedule CDC(c) 2021.
| KEY POINTS |
|
Other Forms of HPV Prevention
HPV infection is the most common sexually transmitted infection (STI) in the United States, and many individuals become infected with multiple types of HPV during their lives CDC 2022. Most HPV infections resolve, become latent, or are not detectable on clinical assays within a few years of exposure and infection Ho, et al. 1998; Moscicki, et al. 1998; Evander, et al. 1995. HPV is transmitted via skin-to-skin contact, so barrier protection, such as male/insertive and female/receptive condoms, offers some but not full protection. Because prior identification of HPV infection in a sexual partner is unlikely, limiting the number of sexual partners may reduce but not eliminate an individual’s exposure to HPV Workowski, et al. 2021.
| KEY POINTS |
|
Cervical Cancer Prevention
| RECOMMENDATIONS |
Cervical Cancer Prevention
|
Abbreviations: ART, antiretroviral therapy; HPV, human papillomavirus. Information on tobacco use and cessation: NYSDOH: Information about Tobacco Use, Smoking and Secondhand Smoke; American Academy of Family Physicians: FDA-Approved Medications for Smoking Cessation |
HPV vaccination, sustained access to and adherence to effective ART, and compliance with recommended screening intervals, treatment schedules, and overall sexual and preventive healthcare are critical aspects of preventing cervical cancer in people with HIV. Minimizing gaps in care or refusal of care, with the goal of identifying treatable precancerous lesions (cervical intraepithelial neoplasia [CIN] 3 or greater), coupled with treatment and follow-up is a powerful strategy to decrease the incidence of HPV-related cancers in individuals with HIV USPSTF, et al. 2018; Massad, et al. 2017; Thorsteinsson, et al. 2016.
The 2019 American Society for Colposcopy and Cervical Pathology (ASCCP) Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors and ASCCP Management Guidelines App Quick Start Guide provide extensive discussion of risk and an app for calculating risk. Factors that increase the risk of cervical cancer include older age, HPV type 16 infection, persistent HPV infection, a cytology result of high-grade squamous intraepithelial lesions, a history of CIN 3, or previous cervical cancer.
ART as prevention: Early in the epidemic, women with HIV presented with cervical cancer at later stages, when treatment was less successful Maiman, et al. 1993. Risk of incident cervical cancer for individuals with HIV has declined significantly over the last 20 years Hernandez-Ramirez, et al. 2017; Robbins, et al. 2017. ART has been found to reduce HPV acquisition, improve regression, and decrease rates of cervical disease progression Carlander, et al. 2018; Kelly, et al. 2018; Liu, et al. 2018; Ghebre, et al. 2017; Adler, et al. 2012. Rates of cervical dysplasia in women with HIV who are virally suppressed on ART and have a CD4 count ≥500 cells/mm3 are comparable to rates in women without HIV Silverberg, et al. 2018; Aho, et al. 2017; Massad, et al. 2017. Cervical disease pathogenesis is the same in women with HIV who are virally suppressed and have a CD4 count ≥500 cells/mm3 as for women who do not have HIV Davies, et al. 2015; Kim, et al. 2013; Konopnicki, et al. 2013; Harris, et al. 2005.
Screening: Countries with a national screening program have lower rates of cervical cancer among women with HIV, and some U.S. cohorts have demonstrated comparable rates of invasive cervical cancer in people with or without HIV Massad, et al. 2009. Cervical cancer screening and prompt referral for treatment of precancerous lesions and invasive cervical cancer are most effective when integrated into routine HIV services, ideally within HIV clinics McCormick Viens, et al. 2023. Universal access to comprehensive HIV treatment, health care maintenance, and HPV prevention and cancer screening are critical for reducing the burden of HPV-related cancers in people with HIV Ghebre, et al. 2017.
Tobacco use cessation: Tobacco use is associated with development of genital HPV lesions and disease Gallaway, et al. 2018, and cigarette smoking potentiates the risk for acquisition of high-grade CIN in individuals with HIV Massad(a), et al. 2012. Clinicians should inform patients of the risks of tobacco use and encourage reduction or cessation of use of all tobacco products as a component of prevention of genital HPV disease. For more information on addressing tobacco use with patients, see the NYSDOH AI guideline Substance Use Screening and Risk Assessment in Adults > Management of Low-, Moderate-, and High-Risk Substance Use and NYSDOH resources at TalkToYourPatients.ny.gov.
Screening for Cervical Abnormalities
| RECOMMENDATIONS |
Screening for Cervical Abnormalities
Age-Based Screening
Concomitant Screening for Anal Cancer and STIs
Post-Hysterectomy Cancer Screening
Post-Cervical Excision HPV Testing
|
Abbreviations: AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; STI, sexually transmitted infection. Note:
|
Purpose of Screening
The primary goal of cervical cytology is to identify and treat precancerous lesions—defined as CIN 3, AIS, and in rare cases, invasive cervical cancer Perkins, et al. 2020. Cervical cancer is a relatively rare finding in the United States; nevertheless, identification and treatment are essential and may include more frequent testing, referral for colposcopy and directed biopsy, and subsequent treatment of biopsy-proven histologic abnormalities. CIN 2 has appreciable regression rates Perkins, et al. 2020.
A histology result of CIN 3 or higher is the established surrogate for cancer risk. CIN 3 is a finding of severely atypical cellular changes that encompass greater than two-thirds of the epithelial thickness and include full-thickness lesions. Previous terms for CIN 3 were “severe dysplasia” and “carcinoma in situ.” CIN 3 was chosen instead of CIN 2 because it is a more pathologically reproducible diagnosis Perkins, et al. 2020. The HPV type distribution in CIN 3 lesions more closely approximates that of invasive cervical cancer.
How cervical cytology results are reported: Cervical cytology currently uses the Bethesda Classification System as standard nomenclature to describe abnormal results that may require further follow-up CPBG 2016; Massad, et al. 2013. The Bethesda Classification System (see Box 1, below) also describes the degree of neoplastic change found on biopsy. These naming conventions are not interchangeable (see guideline section Follow-Up of Abnormal Cervical Cytology Results).
| Box 1: Cytologic and Histologic Classifications of Cervical Dysplasia [a] |
Bethesda Classification System (describes cervical cytology results):
Cervical intraepithelial lesion or neoplasia (describes histology obtained at biopsy):
|
|
Note:
|
Who to Screen
Cervical dysplasia is caused by genital HPV, an STI. Consistent with American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines Perkins, et al. 2020, cervical cancer screening in patients <30 years old with HIV who have a cervix should begin within at least 2 years of first receptive sexual activity or by age 21 years Keller, et al. 2012.
Patients ≥65 years old: HIV has been associated with an increased lifetime risk of cervical cancer; therefore, the ASCCP guidelines recommend continued screening in this population beyond age 65 years Perkins, et al. 2020. However, patients and their clinicians may decide to discontinue screening after a shared decision-making assessment of the risks and benefits, possible mitigating factors involved with ongoing risk for HPV infection, and the purpose of screening. Factors to consider include HPV status and risk of acquisition, history of screening results and risk of cervical cancer, the burden of screening and associated follow-up, viral suppression status, comorbidities, and life expectancy DHHS 2021; Massad, et al. 2021; Aserlind, et al. 2017.
Virally suppressed patients: Patients who are virally suppressed and have demonstrated adherence to HIV care and primary care, who have negative cytology and negative HPV test results, no genital or pelvic complaints, do not use tobacco products, and do not have any other cervical cancer risk factors may benefit from cervical screening every 5 years Robbins, et al. 2017. When navigating extended screening intervals or discontinuation of screening based on lifetime prognosis, a compassionate engagement of patient-centered shared decision-making is critical, so that clinician and patient can address lifetime risk of HPV-related disease, goals for screening, cultural and personal values, and benefits of a personalized screening interval that considers each patient’s unique risk scenario.
| KEY POINTS |
|
Transgender individuals: It is important that care providers and facilities establish a safe and welcoming environment for transgender patients UCSF(a) 2016. Approximately one-third of transgender or gender-diverse individuals assigned female sex at birth identify as nonbinary National Center for Transgender Equality 2017. Asking patients to provide details about all gender-affirming and gynecologic surgical procedures will help establish the need for screening for HPV-related cancers (for terminology and definitions related to transgender care, see the University of California San Francisco Center of Excellence for Transgender Health).
Currently, there are no published data on cervical cancer screening and treatment in transgender men. Although the recommendations in this guideline are based on data from studies in cisgender women, this committee supports extrapolation of that data to support recommendations for all adults with HIV who are eligible for cervical screening.
Transgender men who have a uterine cervix are at risk for cervical cancer, yet screening rates are lower in this population than in cisgender women, largely because of barriers to inclusive, informed medical care for transgender people. Testosterone use in transgender men causes vaginal atrophy, which is associated with high rates of cytology results that are classified as “insufficient.” Notation of testosterone use and amenorrhea, when indicated, will facilitate accurate interpretation of cell morphology in transgender men Tabaac, et al. 2018; UCSF(b) 2016; Peitzmeier, et al. 2014.
Transgender women may undergo genital reconstruction, or vaginoplasty, to create a neovagina. There are no studies to support cervical or vaginal screening of a neovagina; however, visual examination to assess symptoms or as part of routine screening is appropriate Fierz, et al. 2019; UCSF(b) 2016; van der Sluis, et al. 2016; Heller 2015.
| KEY POINTS |
|
HPV Testing
HPV testing: HPV testing is used to assist risk stratification as a primary screening tool. The U.S. Food and Drug Administration (FDA) has approved 2 assays for primary HPV testing to be used for screening individuals ≥25 years old in the general population. Currently, there is limited use of this screening strategy for people with HIV in the United States. Research from a retrospective cohort has suggested that primary HPV screening with reflex to 16/18 genotyping in cervical cancer screening for people with HIV may result in fewer colposcopies over the subsequent 1 to 2 years than cytology with HPV testing Strickler, et al. 2020.
HPV cotesting: Cervical cytology with HPV cotesting has been used to extend cervical screening intervals to every 5 years in women without HIV USPSTF, et al. 2018; ACOG 2016; CPBG 2016. However, cervical cytology with HPV cotesting is not indicated for individuals <30 years old because resolution of HPV infection and cervical dysplasia is likely regardless of HIV status USPSTF, et al. 2018; Plummer, et al. 2007; Woodman, et al. 2001. Aggressive treatment of dysplasia from transient HPV infection may damage the cervix, contribute to preterm delivery, and be more harmful than beneficial in this age group Conner, et al. 2014; Bruinsma and Quinn 2011. HPV cotesting is a useful adjunct to cervical cytology in individuals with HIV ≥30 years old Alade, et al. 2017; Castle, et al. 2012; Keller, et al. 2012.
| KEY POINT |
|
Concomitant Screening for Anal Cancer and STIs
Diagnoses of anal cancer are on the rise in the United States among women in the general population; among men who have sex with men, regardless of their HIV status; and among men and women with HIV Islami, et al. 2017; Palefsky 2017; Hessol, et al. 2013. Anal SILs have been associated with concurrent cervical SILs; however, they can also occur independently. Anal cytology should be performed for all individuals ≥35 years old with HIV, including cisgender women Gaisa, et al. 2017; Stier, et al. 2015; Hessol, et al. 2013; Kojic, et al. 2011, with or without cervical abnormalities, according to guidelines for adults with HIV. Regardless of cytology results, it is important that screening for STIs is performed routinely in patients who engage in risk behaviors (for more information, see CDC: 2021 Sexually Transmitted Infections Treatment Guidelines).
Post-Hysterectomy Cancer Screening
After a patient who does not have HIV has had a hysterectomy for benign disease, routine screening for vaginal cancer is not generally recommended. However, because HIV and HPV infection increase the risk of vaginal SILs Bradbury, et al. 2019; Massad(b), et al. 2012, vaginal cytologic testing post-hysterectomy is recommended for patients with HIV Smeltzer, et al. 2016. SILs on the vaginal cuff can recur from a latent anogenital HPV infection or as primary disease post-hysterectomy, not related to previous cervical infection Smeltzer, et al. 2016; Saslow, et al. 2012.
If the indication for hysterectomy in a patient with HIV is not known, screening should be performed as it would be in a patient with an intact cervix. Individuals with HIV who have undergone hysterectomy and have any history of high-grade CIN, AIS, or invasive cervical cancer, regardless of whether the hysterectomy was performed for that disease or subsequently for benign disease, should receive a minimum of 3 consecutive annual HPV tests before long-term surveillance with cervical cytology and HPV testing every 3 years is initiated Perkins, et al. 2020; Khan, et al. 2016.
The FDA has not approved HPV testing for vaginal samples; however, the sensitivity of HPV-based testing seems superior to cytology alone when screening for high-grade SIL after hysterectomy Perkins, et al. 2020; Khan, et al. 2016. Abnormal vaginal screening results should be managed according to colposcopy guidelines for vaginal cytology Perkins, et al. 2020.
Screening for Cervical Dysplasia During Pregnancy
| RECOMMENDATIONS |
Screening for Cervical Dysplasia During Pregnancy
|
Abbreviations: ASC-H, atypical squamous cells, high-grade squamous intraepithelial lesions cannot be excluded; ASC-US, atypical squamous cells of undetermined significance; HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion. |
Pregnant individuals with HIV should undergo cervical cytology and HPV cotesting as appropriate for their age group. Referral to a practitioner skilled and experienced in colposcopy is recommended for all pregnant patients with cervical screening results of persistent ASC-US, ASC-US with HPV, negative cytology with persistently positive HPV, ASC-H, or LSIL or greater.
The natural history, pathogenesis, rate of progression, and prognosis of cervical cancer are not affected by pregnancy. Colposcopy-directed biopsies are generally safe during pregnancy but should be performed only if a lesion appears to be carcinoma in situ or cancer UpToDate 2023. Endocervical curettage, endometrial biopsy, and treatment without biopsy are unacceptable practices during pregnancy. Diagnostic excisional procedure or repeat biopsy is recommended only if cancer is suspected based on cytology, colposcopy, or histology results Perkins, et al. 2020. Shared decision-making that accounts for a patient’s risk of cancer, ongoing monitoring and treatment plan, pregnancy options, and reported values and goals should be applied when managing cervical dysplasia in pregnancy.
Close follow-up of abnormal cytology or colposcopy results is critical in the postpartum period. The colposcopic exam should be performed no earlier than 4 weeks after delivery; however, biopsy during pregnancy should be conducted for any lesions suspicious for cancer to avoid delays in treatment. To prevent loss to follow-up, the clinician can refer for postpartum colposcopy in the antepartum period, making sure to include the provision of ongoing health insurance.
| KEY POINT |
|
Follow-Up of Abnormal Cervical Cytology Results
| RECOMMENDATIONS |
Follow-Up of Abnormal Cervical Cytology Results
|
Abbreviations: AGC, atypical glandular cells; AIS, adenocarcinoma in situ; ASC-H, atypical squamous cells, HSIL cannot be excluded; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion. Notes:
|
Abnormal cervical cytology and referral for colposcopy: Colposcopy with biopsy is the recommended diagnostic test for cervical dysplasia identified through cervical cytology; colposcopy is not used for primary screening. Colposcopy visually locates specific lesions for directed biopsy and histologic diagnosis and has better sensitivity and specificity for SILs than cytology alone. Abnormal cytology results that require colposcopy include:
- ASC-US with high-risk HPV
- ASC-H
- AGC
- LSILs
- HSILs
- Repeated positive high-risk HPV cotest results in the presence of negative cervical cytology
- Repeated ASC-US cytology, regardless of HPV result (see discussion below)
Figure 1, below, describes appropriate follow-up of abnormal cervical cytology results in patients with HIV.
Figure 1: Follow-Up for Abnormal Cervical Cytology Results in Patients With HIV
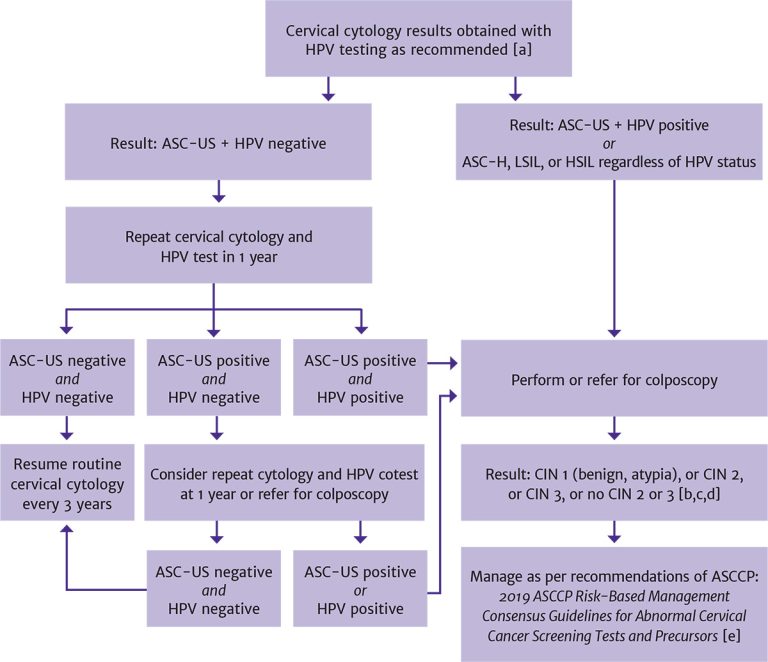
Abbreviations: ASC-H, atypical squamous cells, high-grade squamous intraepithelial lesion cannot be excluded; ASC-US, atypical squamous cells of undetermined significance; ASCCP, American Society for Colposcopy and Cervical Pathology; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Notes:
- In patients <30 years old, HPV reflex testing should be performed in patients with a positive cervical cytology result; in patients ≥30 years old, HPV cotesting is recommended.
- If cotesting was not performed, then HPV reflex testing is indicated following an abnormal cytology result.
- For non–high-grade CIN, refer to ASCCP recommendations for management of LSIL (CIN 1) preceded by ASC-H or HSIL cytology.
- In patients <25 years old, immediate excision is not recommended; in nonpregnant patients ≥25 years old, the decision regarding expedited treatment versus colposcopy with biopsy should be based on shared decision-making between the patient and clinician.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer
screening tests and cancer precursors. J Low Genit Tract Dis 2020;24(2):102-131. [PMID: 32243307]
Download figure: Follow-Up for Abnormal Cervical Cytology Results in Patients With HIV
ASC-US: Because a cervical cytology result of ASC-US indicates the inability to determine whether the cellular abnormality is benign or high risk, an HPV test in response to the cytology result (HPV reflex testing) is recommended regardless of a patient’s age. The purpose is to identify possible high-risk HPV infection, which, if present, requires follow-up with colposcopy.
Vaginal or cervical infections (e.g., trichomonas, herpes simplex virus, gonorrhea, chlamydia, or bacterial vaginosis) or age-related atrophic changes may be associated with inflammation and abnormal cytology results. In addition to considering an individual’s history of dysplasia, clinicians should consider, screen for, and treat inflammatory conditions in all patients, especially those with a cytology result of ASC-US and a negative HPV test result. After a patient’s inflammatory condition(s) have been treated, clinicians may repeat cytology and HPV cotesting before referring to colposcopy.
ASC-H: Cervical cytology results may be described as ASC-US when the lesion cannot be determined to be high grade; however, a result of ASC-H suggests that a lesion is precancerous, and colposcopy is indicated regardless of the HPV cotest result.
LSILs: A cytology result of LSIL indicates early cell changes associated with HPV infection. In women who do not have HIV, LSILs tend to be associated with transient changes that regress over time UpToDate 2023; Solomon, et al. 2002. Data on women with HIV indicate higher rates of recurrence and progression of LSILs than observed among those without HIV Zeier, et al. 2012; Nappi, et al. 2005; Robinson, et al. 2003. Individuals with HIV and LSIL on cytology should be referred for colposcopy.
HSILs: A cytology result of HSILs suggests that a lesion is more likely to be precancerous. HSILs are associated with high-risk types of HPV and have a high risk of progression to cervical intraepithelial neoplasia (CIN) or cancer UpToDate 2023. In individuals with HIV, both LSILs and HSILs require close follow-up and referral for colposcopy.
For nonpregnant individuals ≥25 years old with HSILs, current American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines for the general population recommend consideration of immediate treatment—destruction or excision of precancerous lesions—in cases in which the risk of CIN 3 or higher is ≥25% Perkins, et al. 2020. Treatment without colposcopy removes an intermediate step for those at highest risk for CIN 3 or higher. In cases in which the risk of CIN 3 or higher exceeds 60%, expedited treatment is preferred. Reasons for consideration of expedited treatment will vary and may include limited access to healthcare. Immediate treatment without histologic confirmation is not recommended for individuals <25 years old. The age cutoff of 25 years balances the benefits and harms related to very low cervical cancer rates and high rates of HSIL regression in individuals <25 years old Perkins, et al. 2020. Because individuals with HIV are known to have an elevated risk of cervical cancer, a thorough discussion with patients of the risks and benefits of treatment of cervical dysplasia is crucial to ensuring shared decision-making.
CIN: As described in the guideline section Screening for Cervical Abnormalities, SILs are cytologic findings and CIN is a histologic finding found on biopsy performed at the time of colposcopy.
CIN 1 is used to describe a low-grade lesion and refers to mildly atypical cellular changes in the lower third of the epithelium; HPV cytopathic effect (koilocytotic atypia) is often present.
CIN 2 (formerly called moderate dysplasia) describes a high-grade lesion and refers to atypical cellular changes confined to the basal two-thirds of the epithelium with preservation of epithelial maturation. However, CIN 2 has poor reproducibility and is likely a heterogeneous mix that includes lesions that could be called CIN 1 or 3. CIN 2 is stratified according to p16 immunostaining to identify precancerous lesions. Specimens that are p16 negative are referred to as LSILs and those that are p16 positive are referred to as HSILs. Because of the poor reproducibility of CIN 2, CIN 2 and 3 are often classified together as “CIN 2/3.”
CIN 3 (formerly called severe dysplasia or carcinoma in situ) describes a high-grade lesion and refers to severely atypical cellular changes encompassing greater than two-thirds of the epithelial thickness and includes full-thickness lesions UpToDate 2023.
Indications for expedited treatment: ASCCP guidelines urge consideration of diagnostic excision and treatment without the intermediary step of colposcopy in cases in which risk of CIN 3 exceeds 25%; expedited treatment is preferred when risk exceeds 60% Perkins, et al. 2020. The ASCCP guidelines do not specifically address individuals with HIV (for discussion of risk calculation, see the 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors).
AGC: Any cervical cytology result of AGC requires immediate follow-up with colposcopy and further evaluation (see Figure 2, below). Treatment decisions are based on the resulting tissue diagnosis.
Glandular carcinoma of the cervix may be preceded by a negative cytology result or a test result indicating the presence of AGC Moukarzel, et al. 2017. A cytology result of AGC may indicate a precursor lesion for a glandular cell cervical cancer, although this is rare. AGC may be related to HPV infection or may be a contaminant from endometrial or fallopian tube cancer.
Figure 2: Follow-Up for Cervical Cytology Result of AGC in Patients With HIV [a]
![Figure 2: Follow-Up for Cervical Cytology Result of AGC in Patients With HIV [a]](https://www.hivguidelines.org/wp-content/uploads/2023/07/NYSDOH-AI-Screening-for-Cervical-Dysplasia-and-Cancer-in-Adults-With-HIV-Figure-2_7-20-2023_HG-768x650.jpg)
Abbreviations: AGC, atypical glandular cells; ASCCP, American Society for Colposcopy and Cervical Pathology; HPV, human papillomavirus.
Notes:
- Adapted with permission from the ASCCP. See Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24(2):102-131. [PMID: 32243307]
- Conditions that increase risk for endometrial neoplasia include abnormal uterine bleeding, obesity, or conditions suggesting chronic anovulation.
Download figure: Follow-Up for Cervical Cytology Result of AGC in Patients With HIV
| KEY POINTS |
|
Management of Cervical Cancer
| RECOMMENDATIONS |
Management of Cervical Cancer
|
Individuals with cervical cancer may have few and nonspecific symptoms; when they do present with symptoms, more advanced disease is often found. Vaginal bleeding and postcoital bleeding are the most common symptoms. Malodorous vaginal discharge, pelvic pain, back pain, and lower abdominal pain are also common. Weight loss, leg pain, edema, and obstructive uropathy indicate advanced disease ACS 2020. Patients with a diagnosis of cervical cancer, with or without symptoms, should be referred immediately for assessment and management of their disease. Support services often facilitate patient engagement and maintenance in cancer treatment and care.
The standard therapeutic approach to treating cervical cancer in individuals with HIV is the same for individuals without HIV. Treatment by high-volume surgeons at high-volume hospitals with higher rates of guideline-based care is associated with better cervical cancer survival outcomes Bonte, et al. 2019; Liu, et al. 2018; Uppal, et al. 2017; ACOG 2016; Showalter, et al. 2016. Appropriate staging, management, and therapy for cervical cancer should be determined by a gynecologic oncologist or a clinician with similar training and experience. Although the effect of treatment by gynecologic oncology specialists has not been studied among patients with cervical cancer, research suggests that patients with other gynecologic cancers experience better survival outcomes, especially when treated at National Cancer Institute Comprehensive Cancer Centers Bonte, et al. 2019; Wright, et al. 2017; Bristow, et al. 2015; Minig, et al. 2015; Mercado, et al. 2010.
Management and therapy should be based on the stage of disease. Treatment may include cone biopsy/loop electrosurgical excision, total hysterectomy, radical hysterectomy, radiation therapy, chemotherapy, and combined modality therapy with surgery, radiation, and chemotherapy. The increased risk of treatment failure and high recurrence rate in individuals with HIV demand close follow-up by a multidisciplinary team of clinicians even after definitive treatment for cervical cancer.
All Recommendations
| ALL RECOMMENDATIONS |
HPV Prevention
Cervical Cancer Prevention
Screening for Cervical Abnormalities
Age-Based Screening
Concomitant Screening for Anal Cancer and STIs
Post-Hysterectomy Cancer Screening
Post-Cervical Excision HPV Testing
Screening for Cervical Dysplasia During Pregnancy
Follow-Up of Abnormal Cervical Cytology Results
Management of Cervical Cancer
|
Abbreviations: AGC, atypical glandular cells; AIS, adenocarcinoma in situ; ART, antiretroviral therapy; ASC-H, atypical squamous cells, high-grade squamous intraepithelial lesions cannot be excluded; ASC-US, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; STI, sexually transmitted infection. Information on tobacco use and cessation: NYSDOH: Information about Tobacco Use, Smoking and Secondhand Smoke; American Academy of Family Physicians: FDA-Approved Medications for Smoking Cessation Notes:
|
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making

Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
ACOG. Practice bulletin no. 167: gynecologic care for women and adolescents with human immunodeficiency virus. Obstet Gynecol 2016;128(4):e89-110. [PMID: 27661659]
ACOG. Human papillomavirus vaccination: ACOG committee opinion, number 809. Obstet Gynecol 2020;136(2):e15-21. [PMID: 32732766]
ACS. Signs and symptoms of cervical cancer. 2020 Jan 3. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/signs-symptoms.html [accessed 2022 Mar 22]
ACS. Key statistics for cervical cancer. 2022 Jan 12. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html [accessed 2022 Mar 22]
Adler D. H., Kakinami L., Modisenyane T., et al. Increased regression and decreased incidence of human papillomavirus-related cervical lesions among HIV-infected women on HAART. AIDS 2012;26(13):1645-52. [PMID: 22555167]
Aho I., Kivela P., Haukka J., et al. Declining prevalence of cytological squamous intraepithelial lesions of the cervix among women living with well-controlled HIV - most women living with HIV do not need annual PAP smear screening. Acta Obstet Gynecol Scand 2017;96(11):1330-37. [PMID: 28832899]
Alade R. O., Vragovic O., Duffy C., et al. Human papillomavirus co-testing results effectively triage normal cervical cytology in HIV-positive women aged 30 years and older. J Low Genit Tract Dis 2017;21(2):125-28. [PMID: 28257290]
Aserlind A., Maguire K., Duthely L., et al. Women living with HIV over age of 65: cervical cancer screening in a unique and growing population. Infect Dis Obstet Gynecol 2017;2017:2105061. [PMID: 29075090]
Bonte A. S., Luyckx A., Wyckmans L., et al. Quality indicators for the management of endometrial, cervical and ovarian cancer. Eur J Surg Oncol 2019;45(4):528-37. [PMID: 30337202]
Bradbury M., Xercavins N., Garcia-Jimenez A., et al. Vaginal intraepithelial neoplasia: clinical presentation, management, and outcomes in relation to HIV infection status. J Low Genit Tract Dis 2019;23(1):7-12. [PMID: 30161052]
Bristow R. E., Chang J., Ziogas A., et al. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg 2015;220(5):940-50. [PMID: 25840536]
Bruinsma F. J., Quinn M. A. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG 2011;118(9):1031-41. [PMID: 21449928]
Carlander C., Wagner P., van Beirs A., et al. Suppressive antiretroviral therapy associates with effective treatment of high-grade cervical intraepithelial neoplasia. AIDS 2018;32(11):1475-84. [PMID: 29746299]
Castle P. E., Fetterman B., Poitras N., et al. Safety against cervical precancer and cancer following negative human papillomavirus and Papanicolaou test results in human immunodeficiency virus-infected women. Arch Intern Med 2012;172(13):1041-43. [PMID: 22641193]
CDC. Chapter 5: Human papillomavirus. Manual for the surveillance of vaccine-preventable diseases. 2022 Mar 9. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html [accessed 2022 Mar 22]
CDC(a). Epidemiology and prevention of vaccine-preventable diseases: human papillomavirus. 2021 Aug 18. https://www.cdc.gov/vaccines/pubs/pinkbook/hpv.html [accessed 2022 Mar 22]
CDC(b). HPV and cancer: HPV-associated cancer statistics. 2021 Dec 13. https://www.cdc.gov/cancer/hpv/statistics/index.htm [accessed 2022 Mar 22]
CDC(c). HPV vaccine schedule and dosing. 2021 Nov 1. https://www.cdc.gov/hpv/hcp/schedules-recommendations.html [accessed 2022 Mar 22]
CDC(d). U.S. cancer statistics data visualizations tool, based on 2020 submission data (1999-2018). 2021 Jun. https://gis.cdc.gov/Cancer/USCS/#/StateCounty/ [accessed 2022 Mar 22]
Chaturvedi A. K., Engels E. A., Pfeiffer R. M., et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29(32):4294-4301. [PMID: 21969503]
Clifford G. M., Tully S., Franceschi S. Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin Infect Dis 2017;64(9):1228-35. [PMID: 28199532]
Conner S. N., Frey H. A., Cahill A. G., et al. Loop electrosurgical excision procedure and risk of preterm birth: a systematic review and meta-analysis. Obstet Gynecol 2014;123(4):752-61. [PMID: 24785601]
CPBG. Practice bulletin no. 168: cervical cancer screening and prevention. Obstet Gynecol 2016;128(4):e111-30. [PMID: 27661651]
Davies O., Rajamanoharan S., Balachandran T. Cervical screening in HIV-positive women in the East of England: recent CD4 as the predictive risk factor. Int J STD AIDS 2015;26(13):945-50. [PMID: 25505037]
DHHS. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. 2021 Dec 16. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/human-papillomavirus-disease?view=full [accessed 2022 Mar 22]
Di Donato V., Caruso G., Petrillo M., et al. Adjuvant HPV vaccination to prevent recurrent cervical dysplasia after surgical treatment: a meta-analysis. Vaccines (Basel) 2021;9(5). [PMID: 33919003]
Dryden-Peterson S., Bvochora-Nsingo M., Suneja G., et al. HIV infection and survival among women with cervical cancer. J Clin Oncol 2016;34(31):3749-57. [PMID: 27573661]
Evander M., Edlund K., Gustafsson A., et al. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis 1995;171(4):1026-30. [PMID: 7706782]
Falcaro M., Castanon A., Ndlela B., et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet 2021;398(10316):2084-92. [PMID: 34741816]
FDA. Vaccines, blood & biologics: Gardasil 9. 2020 Aug 21. https://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm426445.htm [accessed 2022 Mar 22]
Fierz R., Ghisu G. P., Fink D. Squamous carcinoma of the neovagina after male-to-female reconstruction surgery: a case report and review of the literature. Case Rep Obstet Gynecol 2019;2019:4820396. [PMID: 30775041]
Gaisa M., Ita-Nagy F., Sigel K., et al. High rates of anal high-grade squamous intraepithelial lesions in HIV-infected women who do not meet screening guidelines. Clin Infect Dis 2017;64(3):289-94. [PMID: 27965301]
Gallaway M. S., Henley S. J., Steele C. B., et al. Surveillance for cancers associated with tobacco use - United States, 2010-2014. MMWR Surveill Summ 2018;67(12):1-42. [PMID: 30383737]
Ghebre R. G., Grover S., Xu M. J., et al. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep 2017;21:101-8. [PMID: 28819634]
Glidden D. V., Amico K. R., Liu A. Y., et al. Symptoms, side effects and adherence in the iPrEx Open-Label Extension. Clin Infect Dis 2016;62(9):1172-77. [PMID: 26797207]
Grabar S., Hleyhel M., Belot A., et al. Invasive cervical cancer in HIV-infected women: risk and survival relative to those of the general population in France. Results from the French Hospital Database on HIV (FHDH)-Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS) CO4 cohort study. HIV Med 2019;20(3):222-29. [PMID: 30693646]
Grulich A. E., van Leeuwen M. T., Falster M. O., et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370(9581):59-67. [PMID: 17617273]
Guan P., Howell-Jones R., Li N., et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131(10):2349-59. [PMID: 22323075]
Hariri S., Unger E. R., Powell S. E., et al. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 2012;206(12):1878-86. [PMID: 23045628]
Harris T. G., Burk R. D., Palefsky J. M., et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA 2005;293(12):1471-76. [PMID: 15784870]
Heller D. S. Lesions of the neovagina--a review. J Low Genit Tract Dis 2015;19(3):267-70. [PMID: 26111041]
Hernandez-Ramirez R. U., Shiels M. S., Dubrow R., et al. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017;4(11):e495-504. [PMID: 28803888]
Hessol N. A., Holly E. A., Efird J. T., et al. Concomitant anal and cervical human papillomavirus infections and intraepithelial neoplasia in HIV-infected and uninfected women. AIDS 2013;27(11):1743-51. [PMID: 23803793]
Ho G. Y., Bierman R., Beardsley L., et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338(7):423-28. [PMID: 9459645]
ICESCC. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer 2007;120(4):885-91. [PMID: 17131323]
Islami F., Ferlay J., Lortet-Tieulent J., et al. International trends in anal cancer incidence rates. Int J Epidemiol 2017;46(3):924-38. [PMID: 27789668]
Karimi-Zarchi M., Allahqoli L., Nehmati A., et al. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020;20(1):274. [PMID: 32106837]
Keller M. J., Burk R. D., Massad L. S., et al. Cervical precancer risk in HIV-infected women who test positive for oncogenic human papillomavirus despite a normal pap test. Clin Infect Dis 2015;61(10):1573-81. [PMID: 26187020]
Keller M. J., Burk R. D., Xie X., et al. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA 2012;308(4):362-69. [PMID: 22820789]
Kelly H., Weiss H. A., Benavente Y., et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV 2018;5(1):e45-58. [PMID: 29107561]
Kemp T. J., Hildesheim A., Safaeian M., et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine 2011;29(11):2011-14. [PMID: 21241731]
Khan M. J., Massad L. S., Kinney W., et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol 2016;141(2):364-70. [PMID: 26915529]
Kim S. C., Messing S., Shah K., et al. Effect of highly active antiretroviral therapy (HAART) and menopause on risk of progression of cervical dysplasia in human immune-deficiency virus- (HIV-) infected women. Infect Dis Obstet Gynecol 2013;2013:784718. [PMID: 24453469]
Kojic E. M., Cu-Uvin S., Conley L., et al. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study). Sex Transm Dis 2011;38(4):253-59. [PMID: 20966828]
Kojic E. M., Kang M., Cespedes M. S., et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014;59(1):127-35. [PMID: 24723284]
Konopnicki D., Manigart Y., Gilles C., et al. Sustained viral suppression and higher CD4+ T-cell count reduces the risk of persistent cervical high-risk human papillomavirus infection in HIV-positive women. J Infect Dis 2013;207(11):1723-29. [PMID: 23463709]
Lichter K., Krause D., Xu J., et al. Adjuvant human papillomavirus vaccine to reduce recurrent cervical dysplasia in unvaccinated women: a systematic review and meta-analysis. Obstet Gynecol 2020;135(5):1070-83. [PMID: 32282601]
Liu G., Sharma M., Tan N., et al. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018;32(6):795-808. [PMID: 29369827]
Maiman M., Fruchter R. G., Guy L., et al. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer 1993;71(2):402-6. [PMID: 8093678]
Massad L. S., Einstein M. H., Huh W. K., et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121(4):829-46. [PMID: 23635684]
Massad L. S., Hessol N. A., Darragh T. M., et al. Cervical cancer incidence after up to 20 years of observation among women with HIV. Int J Cancer 2017;141(8):1561-65. [PMID: 28670714]
Massad L. S., Seaberg E. C., Watts D. H., et al. Long-term incidence of cervical cancer in women with human immunodeficiency virus. Cancer 2009;115(3):524-30. [PMID: 19127538]
Massad L. S., Xie X., Minkoff H. L., et al. Frequency of high grade squamous cervical lesions among women over age 65 years living with the human immunodeficiency virus. Am J Obstet Gynecol 2021;225(4):411.e1-7. [PMID: 33957115]
Massad(a) L. S., Xie X., Greenblatt R. M., et al. Effect of human immunodeficiency virus infection on the prevalence and incidence of vaginal intraepithelial neoplasia. Obstet Gynecol 2012;119(3):582-89. [PMID: 22353957]
Massad(b) L. S., D'Souza G., Tian F., et al. Negative predictive value of Pap testing: implications for screening intervals for women with human immunodeficiency virus. Obstet Gynecol 2012;120(4):791-97. [PMID: 22996096]
McClymont E., Lee M., Raboud J., et al. Prevalent and persistent oncogenic HPV types in a cohort of women living with HIV prior to HPV vaccination. Int J Gynaecol Obstet 2020;150(1):108-15. [PMID: 32342504]
McCormick Viens L. J., Godfrey C., Adeoye O., et al. Progress towards the elimination of cervical cancer among women living with HIV. Abstract 636. CROI; 2023 Feb 19-22. https://www.croiconference.org/abstract/progress-towards-the-elimination-of-cervical-cancer-among-women-living-with-hiv/
McKenzie N. D., Kobetz E. N., Hnatyszyn J., et al. Women with HIV are more commonly infected with non-16 and -18 high-risk HPV types. Gynecol Oncol 2010;116(3):572-77. [PMID: 19906410]
Mercado C., Zingmond D., Karlan B. Y., et al. Quality of care in advanced ovarian cancer: the importance of provider specialty. Gynecol Oncol 2010;117(1):18-22. [PMID: 20106512]
Minig L., Padilla-Iserte P., Zorrero C. The relevance of gynecologic oncologists to provide high-quality of care to women with gynecological cancer. Front Oncol 2015;5:308. [PMID: 26835417]
Money D. M., Moses E., Blitz S., et al. HIV viral suppression results in higher antibody responses in HIV-positive women vaccinated with the quadrivalent human papillomavirus vaccine. Vaccine 2016;34(40):4799-4806. [PMID: 27544584]
Moscicki A. B., Ellenberg J. H., Farhat S., et al. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis 2004;190(1):37-45. [PMID: 15195241]
Moscicki A. B., Shiboski S., Broering J., et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998;132(2):277-84. [PMID: 9506641]
Moukarzel L. A., Angarita A. M., VandenBussche C., et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genit Tract Dis 2017;21(2):91-96. [PMID: 27977543]
Nappi L., Carriero C., Bettocchi S., et al. Cervical squamous intraepithelial lesions of low-grade in HIV-infected women: recurrence, persistence, and progression, in treated and untreated women. Eur J Obstet Gynecol Reprod Biol 2005;121(2):226-32. [PMID: 16054967]
National Center for Transgender Equality. The report of the 2015 U.S. Transgender Survey. 2017 Dec 17. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf [accessed 2022 Mar 22]
Nayar R., Wilbur D. C. The Pap test and Bethesda 2014. Cancer Cytopathol 2015;123(5):271-81. [PMID: 25931431]
NCI SEER. Cancer statistics review, 1975-2014. 2018 Apr 2. https://seer.cancer.gov/archive/csr/1975_2014/ [accessed 2022 Mar 22]
Orlando G., Bianchi S., Fasolo M. M., et al. Cervical human papillomavirus genotypes in HIV-infected women: a cross-sectional analysis of the VALHIDATE study. J Prev Med Hyg 2017;58(4):e259-65. [PMID: 29707656]
Palefsky J. M. Human papillomavirus-associated anal and cervical cancers in HIV-infected individuals: incidence and prevention in the antiretroviral therapy era. Curr Opin HIV AIDS 2017;12(1):26-30. [PMID: 27828801]
Peitzmeier S. M., Reisner S. L., Harigopal P., et al. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: implications for cervical cancer screening. J Gen Intern Med 2014;29(5):778-84. [PMID: 24424775]
Perkins R. B., Guido R. S., Castle P. E., et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24(2):102-31. [PMID: 32243307]
Petrosky E., Bocchini J. A., Hariri S., et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2015;64(11):300-304. [PMID: 25811679]
Plummer M., Schiffman M., Castle P. E., et al. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis 2007;195(11):1582-89. [PMID: 17471427]
Robbins H. A., Strickler H. D., Massad L. S., et al. Cervical cancer screening intervals and management for women living with HIV: a risk benchmarking approach. AIDS 2017;31(7):1035-44. [PMID: 28323758]
Robinson W. R., Luck M. B., Kendall M. A., et al. The predictive value of cytologic testing in women with the human immunodeficiency virus who have low-grade squamous cervical lesions: a substudy of a randomized, phase III chemoprevention trial. Am J Obstet Gynecol 2003;188(4):896-900. [PMID: 12712082]
Saslow D., Solomon D., Lawson H. W., et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012;62(3):147-72. [PMID: 22422631]
Showalter T. N., Camacho F., Cantrell L. A., et al. Determinants of quality care and mortality for patients with locally advanced cervical cancer in Virginia. Medicine (Baltimore) 2016;95(8):e2913. [PMID: 26937934]
Silverberg M. J., Leyden W. A., Chi A., et al. Human immunodeficiency virus (HIV)- and non-HIV-associated immunosuppression and risk of cervical neoplasia. Obstet Gynecol 2018;131(1):47-55. [PMID: 29215531]
Smeltzer S., Yu X., Schmeler K., et al. Abnormal vaginal Pap test results after hysterectomy in human immunodeficiency virus-infected women. Obstet Gynecol 2016;128(1):52-57. [PMID: 27275815]
Solomon D., Davey D., Kurman R., et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA 2002;287(16):2114-19. [PMID: 11966386]
Stier E. A., Sebring M. C., Mendez A. E., et al. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: a systematic review. Am J Obstet Gynecol 2015;213(3):278-309. [PMID: 25797230]
Strickler H. D., Keller M. J., Hessol N. A., et al. Primary HPV and molecular cervical cancer screening in US women living with HIV. Clin Infect Dis 2020;72(9):1529-37. [PMID: 32881999]
Tabaac A. R., Sutter M. E., Wall C. S., et al. Gender identity disparities in cancer screening behaviors. Am J Prev Med 2018;54(3):385-93. [PMID: 29338956]
Thorsteinsson K., Ladelund S., Jensen-Fangel S., et al. Incidence of cervical dysplasia and cervical cancer in women living with HIV in Denmark: comparison with the general population. HIV Med 2016;17(1):7-17. [PMID: 26058995]
UCSF(a). Transgender care & treatment guidelines: creating a safe and welcoming clinic environment. 2016 Jun 17. https://transcare.ucsf.edu/guidelines/clinic-environment [accessed 2022 Mar 9]
UCSF(b). Transgender care & treatment guidelines: screening for cervical cancer in transgender men. 2016 Jun. http://transhealth.ucsf.edu/trans?page=guidelines-cervical-cancer [accessed 2022 Mar 22]
Uppal S., Chapman C., Spencer R. J., et al. Association of hospital volume with racial and ethnic disparities in locally advanced cervical cancer treatment. Obstet Gynecol 2017;129(2):295-304. [PMID: 28079775]
UpToDate. Cervical cancer screening: risk assessment, evaluation, and management after screening. 2023 Jun 21. https://www.uptodate.com/contents/cervical-cancer-screening-risk-assessment-evaluation-and-management-after-screening [accessed 2022 Mar 22]
USPSTF, Curry S. J., Krist A. H., et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320(7):674-86. [PMID: 30140884]
van der Sluis W. B., Buncamper M. E., Bouman M. B., et al. Prevalence of neovaginal high-risk human papillomavirus among transgender women in The Netherlands. Sex Transm Dis 2016;43(8):503-5. [PMID: 27414682]
Wheeler C. M., Castellsague X., Garland S. M., et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012;13(1):100-110. [PMID: 22075170]
Wilkin T. J., Chen H., Cespedes M. S., et al. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS Clinical Trials Group Protocol A5298. Clin Infect Dis 2018;67(9):1339-46. [PMID: 29659751]
Winer R. L., Hughes J. P., Feng Q., et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med 2006;354(25):2645-54. [PMID: 16790697]
Woodman C. B., Collins S., Winter H., et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet 2001;357(9271):1831-36. [PMID: 11410191]
Workowski K. A., Bachmann L. H., Chan P.A., et al. Sexually transmitted diseases treatment guidelines, 2021. MMWR Recomm Rep 2021;70(RR-4):1-187. [PMID: 26042815]
Wright T. C., Behrens C. M., Ranger-Moore J., et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol 2017;144(1):51-56. [PMID: 28094038]
Zeier M. D., Botha M. H., van der Merwe F. H., et al. Progression and persistence of low-grade cervical squamous intraepithelial lesions in women living with human immunodeficiency virus. J Low Genit Tract Dis 2012;16(3):243-50. [PMID: 22460273]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | November 15, 2017 |
| Date of current publication | March 25, 2022 |
| Highlights of changes, additions, and updates in the March 25, 2022 edition |
Comprehensive update |
| Intended users | Clinicians in New York State who provide primary, HIV, and gynecological care to adults with HIV who are at risk of developing cervical dysplasia or cancer associated with human papillomavirus infection |
| Lead author(s) |
Maria Teresa Timoney, MS, RN, CNM; Jessica M. Atrio, MD, MSc |
| Writing group |
Joseph P. McGowan, MD, FACP, FIDSA; Steven M. Fine, MD, PhD; Rona Vail, MD; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD |
| Author and writing group conflict of interest disclosures |
Joseph P. McGowan, MD, FACP, FIDSA: Institutional Pharma grant recipient/support, clinical trial; Gilead |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines | |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on January 17, 2024

